Chemical reactions is the main focus of chemistry because that's where the action is. That's when something gets made, changed, or destroyed. So a lot of emphasis is placed on chemical reactions.
Chemical reactions can be understood by these three areas of focus. A reaction is basically shuffling around the building blocks of chemistry (atoms/ions/molecules/electrons). Force & energy is what decides if the reaction occurs and how fast. Mathematics helps you keep an inventory of all the starting and ending materials.
Synthesis (Combination)
Decomposition
Single Replacement/Displacement
Double Replacement/Displacement
Oxidation (Combustion)
1. Review the general pattern for double replacement reactions
2. Predict if a reaction will occur based on a few simple rules.
3. Carry out several double-replacement reactions used for various applications
a. Tap Water Purification: Removal of iron ions using a double replacement reaction
b. Drinking Water Purification: Removal of copper ions using a double replacement reaction
c. Photography: Synthesis of several light sensitive silver halides using a double replacement reaction.
d. A double-replacement reaction because of an unstable product.

General Pattern for Double Replacement Reactions:
Double Replacement Pattern: AB + CD → AD + CB
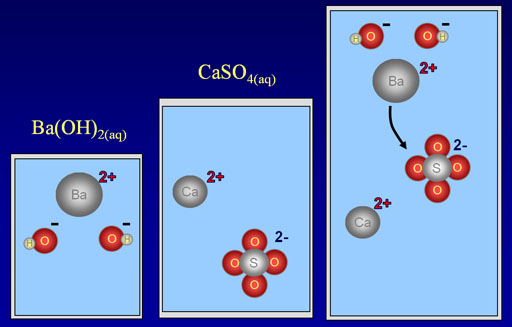
Two compounds exchange ions to form two new compounds. In the generic compound "AB", the "A" element, which is usually a metal, exchanges places with another metal in compound "CD". To the right side of the arrow shows their final arrangement.
Note: The letters are just letters that represent any ion. The "B" is not boron and "C" is not carbon.
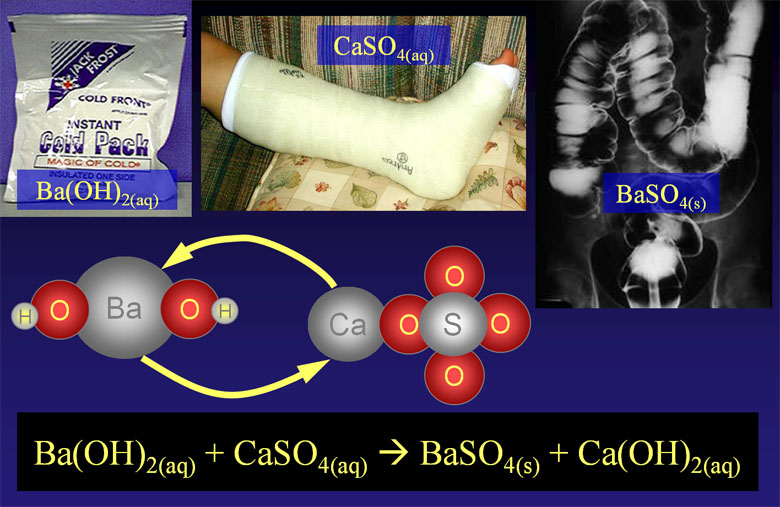
As a specific example, we are showing barium hydroxide, Ba(OH)2, reacting with calcium sulfate, CaSO4. When the reaction occurs, the barium ion (Ba2+) will take the place of the calcium ion (Ca2+).
Below is the full ionic equation:
Ba2+(aq) + 2OH-(aq) + Ca2+(aq)+ SO42-(aq)→ BaSO4(s) + Ca2+(aq)+ 2OH-(aq)
If we remove the ions that didn't react (the spectator ions), we get the below net reaction:
Ba2+(aq) + SO42-(aq)→ BaSO4(s)
So the only reaction that happened was the positive barium ions bonded with the negative sulfate ions. Bonds that are formed because of opposite charges are called ionic bonds. The only reason this happened is that barium sulfate is not soluble in water. The reason it's not soluble is that the ionic bonds in barium sulfate are too strong for water to pull apart. If you knew that barium sulfate was not soluble in water, you could predict that this reaction will happen. Consulting a table that shows what is soluble or not, will tell you if these type of double-replacement reactions will occur. You can also consult with the rules of solubility.

For single replacement reactions, the reaction took place if the metal was more active (higher on the list) than the metal ion that it was in contact with. Double-replacement reactions take place for different reasons. One reason is based on solubility. In other words, whether or not certain positive ions form a solid (a precipitate) if they contact certain negative ions. Knowing which positive ions will form solids with which negative ions can be learned from a SOLUBILITY TABLE or SOLUBILITY RULES.
If a compound forms a solid precipitate(is insoluble), it will form upon mixing two solutions, one having the positive ion and the other containing the negative ion. Solid silver chloride, for example, precipitates on mixing any solution containing chloride ions with any other solution of silver ions.
"aq" stands for aqueous meaning those two ions are soluble and stay in solution. In other words, there is no reaction (no precipitate) when those ions come in contact with each other.
chloride |
bromide |
iodide |
sulfide |
hydroxide |
carbonate |
nitrate |
sulfite |
sulfate |
phosphate |
acetate |
|
Cl- |
Br- |
I- |
S2- |
OH- |
CO32- |
NO3- |
SO32- |
SO42- |
PO43- |
C2H3O2- |
|
| H+ | aq |
aq |
aq |
solid |
liquid1* |
gas2* |
aq |
gas3* |
aq |
aq |
liquid |
| Na+ | aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
| K+ | aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
aq |
| NH4+ | aq |
aq |
aq |
aq |
gas4* |
aq |
aq |
aq |
aq |
aq |
aq |
| Ag+ | solid |
solid |
solid |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
solid |
| Mg2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Ca2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Ba2+ | aq |
aq |
aq |
solid |
aq |
solid |
aq |
solid |
solid |
solid |
aq |
| Fe2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Fe3+ | aq |
aq |
solid |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
solid |
| Co2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Ni2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Cu2+ | aq |
aq |
solid |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Zn2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Hg2+ | aq |
solid |
solid |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Cd2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Sn2+ | aq |
aq |
solid |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Pb2+ | solid |
solid |
solid |
solid |
solid |
solid |
aq |
solid |
solid |
solid |
aq |
| Mn2+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
| Al3+ | aq |
aq |
aq |
solid |
solid |
solid |
aq |
solid |
aq |
solid |
aq |
The solubility table above can also be described using the below solubility rules.
Solubility Rules:
1. All compounds of Na+, K+,
and NH4+ are soluble.
2. All compounds of NO3- are soluble.
3. All compounds of C2H3O2- are soluble except for silver or iron(III).
4. All compounds of Cl-, Br-,
and I- are soluble except Ag+, Hg22+,
and Pb2+.
5. All compounds of SO42-
are soluble except Ca2+, Ag+, Pb2+, Sr2+,
and Ba2+.
6. All metal hydroxides (OH-) are insoluble except those of Groups IA. Group IIA metal hydroxides are
moderately soluble.
7
. All carbonates (CO32-),
phosphates (PO43-), and sulfides (S2-) are
insoluble except those of Group IA and ammonium (NH4+).
8. All metal oxides (O2-) are
insoluble except those of Group IA, that dissolve to form hydroxides. Oxides
of Group IIA are slightly soluble.

Using the solubility chart and solubility rules, we will see how we can make silver acetate. Acetate is in the far right column. As we come down we see that the silver ion will form a solid (a precipitate) with the acetate ion. That means if we find a salt of silver that is soluble (aqueous) and a salt of acetate that is soluble, then we can mix them and the silver ions will bump into the acetate ions to form the insoluble (solid) silver acetate we want. In the solubility table and #2 of the solubility rules, the only ion that keeps silver dissolved is the nitrate ion. So we locate some silver nitrate (like what's in the kit). Now we look for a soluble (aqueous) salt of acetate. According to our table and #3 of the solubility rules, we can use about any positive ion other than iron(III), Fe3+. For example, sodium acetate, potassium acetate, ammonium acetate, and many others would provide us with the acetate ions we need. This the double-replacement reaction we want. Picking sodium acetate as one source, we see that silver takes the place of sodium (Na) to make silver acetate.
NaC2H3O2(aq) + AgNO3(aq) → AgC2H3O2(s) + NaNO3(aq)
By the way, silver acetate was once used in small quantities (2.5 milligrams) in lozenges and chewing gum to deter smoking. Apparently some ingredient in the cigarette smoke would react with the silver acetate to produce a compound that tasted very bad (a strong metallic taste).
The three most common unstable compounds which give gases are:
Carbonic acid: H2CO3 → CO2(g) + H2O(l)
Sulfurous acid: H2SO3 → SO2(g) + H2O(l)
Ammonium hydroxide: NH4OH → NH3(g) + H2O(l)
For example, if hydrochloric acid and sodium bicarbonate are mixed we get the below reaction.
HCl(aq) + NaHCO3(aq) → H2CO3(l) + NaCl(aq)
You can see that the H in HCl exchanges places with Na in NaHCO3. So this looks like a double-replacement reaction; however, the H2CO3 formed is not a solid that takes itself out of solution. H2CO3 stays in the solution and the H in H2CO3 can easily come off, meaning that H and Na can rather easily go back to where they came from. So there would be no double-replacement. However, H2CO3 is unstable and it will decompose into CO2 gas and H2O. After CO2 gas escapes, it can not longer combine with water to reform H2CO3. With H2CO3 decomposing, it can't give back the hydrogen atom that it took from HCl. That makes the double-replacement reaction possible.

Here is the double-replacement reaction again, but with the follow-up decomposition reaction.
HCl(aq) + NaHCO3(aq) → H2CO3(l) + NaCl(aq)
↓
H2CO3 ® CO2(g) +
H2O(l)
Since H2CO3 decomposes into CO2 and H2O, you can see bubbles forming. That's a sign that the double-replacement reaction is occurring.

Neutralizing Corrosive Chemicals:
Lye (sodium hydroxide) is used to make biodiesel and for unclogging a drain. Let's say a jar of it fell and spilled all over the floor. Pure sodium hydroxide is very corrosive and dangerous. You sweep up what you can, but you need to neutralize the remaining powder that can't be swept up. Using vinegar (dilute acetic acid) to cause a double-replacement reaction will neutralize the sodium hydroxide.
Notice the acidic hydrogen on acetic acid is shown in red. It's called the "acidic hydrogen" because it is the hydrogen which comes off of acetic acid to form H+ ions. That hydrogen ion replaces the sodium ion on sodium hydroxide. It does that because the H+ ion will bond very strongly with the hydroxide (OH-) ion to form water (HOH = H2O).
Note: Sometimes acetic acid is written as CH3COOH. The acidic hydrogen is written last. In that case, the H+ ion is "B" in the generic "AB" compound. However, it still is a double-replacement reaction.


Excess iron in tap water will stain sinks, tubs, showers, and clothes. Certain salts of iron are soluble and some are not. The iron salts that are soluble pass through filters and a stay in the tap water until they come in contact with the air. Then they form the reddish-brown iron stains.
Also, if the level or iron ions are too high, the taste of the water is affected.
One strategy in getting trapping the dissolved iron salts is to change them to iron salts that are not soluble. That way solid iron particles can be filtered out.

Your kit has some iron(II) sulfate. This iron salt does dissolve in water and is one source of iron in tap water.
Your kit also has some sodium carbonate (washing soda). This will provide us with a source of carbonate ions (CO32-() which will combine with the iron ions to form the insoluble iron(II) carbonate.
FeSO4(aq) + Na2CO3(aq) → FeCO3(s) + Na2SO4(aq)
The above reaction shows the double-replacement going on. Iron and sodium swap places. The below reaction shows the total ionic equation, which better reflects what is going on.
Fe2+(aq) + SO42-(aq) + 2Na+(aq) + CO32-(aq) → FeCO3(s) + 2Na+(aq)+ SO42-(aq)
If we don't write the ions that don't react, we get the below simpler reaction, which is iron bumping into carbonate ions and forming solid iron(II) carbonate.
Fe2+(aq) + CO32-(aq) → FeCO3(s)

Use your microspatula to place a little of the iron(II) sulfate powder into a empty clean test tube. Also, place a little sodium carbonate into another empty clean test tube. See outer test tubes in image for approximate amounts.
Note: The iron(II) ion Fe2+ is also referred to as "ferrous". So iron(II) sulfate is also called ferrous sulfate. The iron(III) ion with plus 3 charge (Fe3+) is referred to as "ferric". So iron(III) sulfate would also be called ferric sulfate.
The "-ous" endings were were used for the ion with the lower positive charge. The "-ic" ending was for the ion with the larger positive charge. For example, Cu+ is indicated with "-ous" ending and Cu2+ had the "-ic" ending. So copper(I) chloride is called cuprous chloride, and copper(II) chloride is called cupric chloride.
The use of the "-ous" and "-ic" endings was an earlier nomenclature rule compared to using Roman numerals to express the charge. However, that older nomenclature is still in widespread use.




You should see a blue-green precipitate of iron(II) carbonate. The carbonate salt of iron is not soluble so it forms solid particles in the solution.
Fe2+(aq) + CO32-(aq) → FeCO3(s)
A simple filter like a filter paper will not remove Fe2+ ions. However, when those iron ions are bonded with carbonate ions to form solid iron(II) carbonate, those particles can be filtered out of the water using filter paper.

The iron(II) carbonate precipitate above was formed with a rather concentrated solution of iron sulfate. Iron levels won't be that high in tap water. So let's try a more realistic level of iron that might be in tap water.
Transfer just a small amount of the iron(II) sulfate powder to an empty test tube. See image for approximate amount.
Add water to these few granules of iron(II) sulfate to dissolve them. Fill the test tube to about half full with purified water.

Like before, transfer some of the sodium carbonate solution to the test tube that has just a small amount of dissolved iron(II) sulfate.
For us, the solution went from clear to a light green. There also seems to be some small green particles in the solution.
a1) What did you see when you first added the sodium carbonate solution?

Here we have both precipitates from the strong and weak solutions of iron(II) sulfate.
In a few minutes, the light green color of the weak solution has turned into dark green particles that are starting to settle out. This is good news. This means that even small amounts of iron ions can be turned into solid particles. As particles, they can be trapped using standard filters.
Take a photo of your test tubes that show the reaction of carbonates with both the strong and weak iron(II) sulfate solutions.
a2) Instead of iron(II) sulfate (FeSO4) to make iron(II) carbonate, what other iron compound could have been used as a source of iron ions? (consult the solubility table).
a3) Instead of sodium carbonate (Na2CO3) as the source of carbonate (CO32-) ions, what other carbonate compound could have been used? (consult the solubility table)

Here is a close up of the precipitates. Notice the reddish-orange color. That's probably some of the unreacted iron(II) ions that are reacting with oxygen in the air to form iron oxides and possibly iron hydroxides. These have the characteristic rust color that we associate with iron stains.


Removal of toxic copper ions:
Algae are very hardy plants that are difficult to kill, but copper ions are commonly used to kill algae in pools and ponds.
Excess iron ions in water are more of a nuisance; however, excess copper ions are toxic when ingested. Anything over 0.002 grams of copper ions per liter of water is considered unsafe.
This next experiment takes the same strategy that was used to remove iron ions as the way to take out toxic copper ions.

By now, the copper(II) sulfate crystals should be dissolved. Add some more sodium carbonate to the test tube that already has some dissolved sodium carbonate. Then add some more purified water to dissolve it.
Also transfer a small amount of the dissolved copper(II) sulfate solution to an empty test tube. Add more water to dilute it.
Like before you will add some dissolved sodium carbonate to both strong and weak solution of dissolved copper(II) sulfate. Here is the reaction you should see. It's very similar to the double-replacement we did with iron(II) sulfate and sodium carbonate.
CuSO4(aq) + Na2CO3(aq) → CuCO3(s) + Na2SO4(aq)
The above reaction shows the double-replacement going on. Copper and sodium swap places. The below reaction shows the total ionic equation, which better reflects what is going on.
Cu2+(aq) + SO42-(aq) + 2Na+(aq) + CO32-(aq) → CuCO3(s) + 2Na+(aq)+ SO42-(aq)
If we leave out the ions that don't react, we get the below simpler reaction, which is copper bumping into carbonate ions and forming solid copper(II) carbonate.
Cu2+(aq) + CO32-(aq) → CuCO3(s)

Add some of the sodium carbonate solution to both the strong and weak copper(II) sulfate solutions. Look for any precipitation. The strong solution created a lot of blue-green precipitation made of copper(II) carbonate. Even the weak solution shows precipitation. This is good news. That means these particles of copper(II) carbonate can be trapped with a standard filter. So using this technique can remove the toxic copper ions from tap water.
b1) What did your precipitate of copper(II) carbonate look like?
Our next step is to use a paper filter to trap the particles of copper(II) carbonate.
If a filter wasn't available, allowing the precipitate to settle to the bottom and only using the water above the precipitate would be another way to get water that was free of copper ions.
It seems that having some sodium carbonate might be a handy thing to have on a trip. It can cause several toxic metals in the water to precipitate out.

Get the plastic funnel from the kit and set it in an empty test tube. Also, get a sheet of filter paper.
First fold the filter paper in half.

Then you fold the filter paper again, but not in half. See image below.

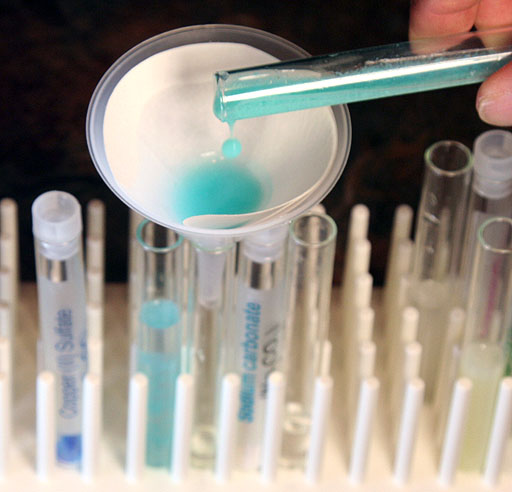
Pour the contents of the test tube that had the sodium carbonate added to the strong copper(II) sulfate solution. That will have the most copper(II) carbonate precipitate.
Take a photo of your filter setup for trapping copper(II) carbonate.
b2) Instead of copper(II) sulfate (CuSO4) as the source of copper ions to make copper(II) carbonate, what other copper compound could have been used as a source of copper ions? (consult the solubility table).
b3) Instead of sodium carbonate (Na2CO3) as the source of carbonate (CO32-) ions, what other carbonate compound could have been used? (consult the solubility table)
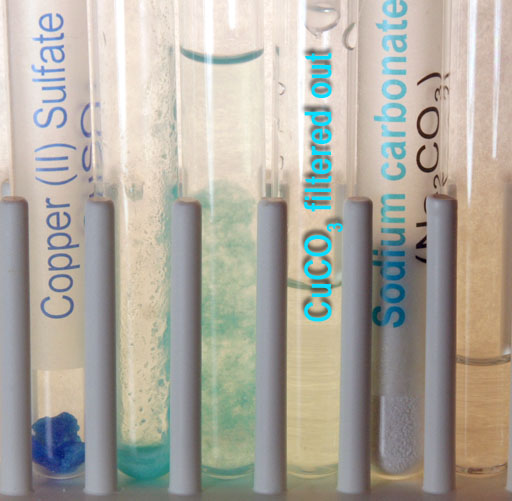
As the solution passes through the filter, it appears that the filter traps the copper(II) carbonate precipitate. The solution that is collected is clear. Warning: If there wasn't enough sodium carbonate added to react with all of the copper(II) sulfate, there will still be copper ions in this solution. Only by adding a little more sodium carbonate solution can one be sure.
b4) Go ahead and add a little more sodium carbonate solution to this liquid that passed through the filter. Did more blue-green precipitate appear?

In lab 4: Physical and Chemical Changes, you learned about silver nitrate and its reaction with sodium chloride. At that time, we didn't mention it was a double-replacement reaction. For this experiment, we will use silver nitrate to produce double-replacement reactions with three different halides (chloride, bromide, and iodide). All of these have been used as the light sensitive compounds that are used in photographs.
The photo, as you may recall, is the first person ever to be photographed. It was back in 1839. The photo was that of the photographer, Robert Cornelius.

Locate the test tubes with sodium chloride, potassium chloride, and potassium iodide. Also, get 3 clean test tubes. If you need to, wash out the ones used earlier in this lab. Use purified water to rinse them, but it is OK if there's a little water left in the test tubes. They don't have to be dry.
Add a small amount of each of these salts to an empty test tube. See photo for amounts that are to be used.
Add purified water to each of these to dissolve the salts. Add enough water to bring the level up to the top of the white plastic posts. That's about 4 mL.
After adding water, shake or swirl the water to dissolve the salts.

Add about 7 drops of silver nitrate to each of the salt solutions.
Here is the double-replacement reaction we expect after adding silver nitrate to dissolved sodium chloride. Sodium takes silver's place and silver takes sodium's place.
NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq)
Because these are dissolved in water, the ions are not together but floating around separately. That is more apparent with a full ionic equation shown below.
Na+(aq) + Cl-(aq) + Ag+(aq)+ NO3-(aq)→ AgCl(s) + Na+(aq)+ NO3-(aq)

If we leave out the ions that don't react, we get the below simpler reaction, which is silver ions bumping into sodium ions and forming solid silver chloride.
Ag+(aq)+ Cl-(aq))→ AgCl(s)
Doing the same for potassium bromide and potassium iodide.
Ag+(aq)+ Br-(aq))→ AgBr(s)
Ag+(aq)+I-(aq))→ AgI(s)
Take a photo of your silver halide precipitates.

There is a bit of a color difference between these silver halides.
c1) What color would you call the silver chloride precipitate?
c2) What color would you call the silver bromide precipitate?
c3) What color would you call the silver iodide precipitate?
If exposed to the sunlight (or UV light), all of these will become black in color due to the silver ion breaking away from the silver salt to form small particles of silver metal.
c4) Instead of sodium chloride (NaCl) as a source of chloride ions to make silver chloride, what other chloride compound could have been used as a source of chloride ions? (consult the solubility table).
c5) Instead of potassium bromide (KBr) as the source of bromide (Br-) ions to make silver bromide, what other bromide compound could have been used? (consult the solubility table)
c6) Instead of potassium iodide (KI) as the source of iodide (I-) ions to make silver iodide, what other iodide compound could have been used? (consult the solubility table)

In the previous experiments of this lab, the double-replacement occurred because one of the products formed a precipitate, which prevents the reaction from reversing. Earlier it was mentioned that another way a double-replacement reaction would proceed is if one of the products decomposes into a gas and water. The decomposition prevents the reaction from reversing.
Locate the 0.1M HCl solution and the test tube with sodium bicarbonate (NaHCO3). Transfer a small amount of the sodium bicarbonate to an empty test tube. See image for amount to use.
This time don't add any water to the sodium bicarbonate that you transferred. There is already water in the 0.1 M HCl.

Add 0.1 M HCl to the sodium bicarbonate powder.
Bring the level up to about halfway up the white posts.

Here is the double-replacement reaction with the follow-up decomposition reaction.
HCl(aq) + NaHCO3(aq) → H2CO3(l) + NaCl(aq)
↓
H2CO3 ® CO2(g) +
H2O(l)
Since H2CO3 decomposes into CO2 and H2O, you can see CO2 bubbles forming. That's a sign that the double-replacement reaction is occurring.
d1) Instead of hydrochloric acid (HCl) as a source of hydrogen ions (H+)to make carbonic acid (H2CO3), what other acid could have been used as a source of hydrogen ions? (consult the solubility table).
If you wish, you can copy the below summary into your email (or Word document) and type your answers after the descriptions. The required photos can either be attached to the email or inserted in the Word document if going that route. Try to keep each image under 2 megabytes. If the first letter of your last name is between A and G, send your lab reports to Loree Cantrell-Briggs at Loree.Cantrell-Briggs@phoenixcollege.edu If the first letter of your last name is between H and Z, send your lab reports to Quinn Thacker at QRT2004@yahoo.com. . Be sure to title the email "Lab 9".
a. Lab 9 Experiment 1: Tap Water Treatment: Removal of iron ions
a1) What did you see when you first added the sodium carbonate solution?
Attach a photo of your test tubes that show the reaction of sodium carbonate with both the strong and weak iron(II) sulfate solutions.
a2) Instead of iron(II) sulfate (FeSO4) to make iron(II) carbonate, what other iron compound could have been used as a source of iron ions? (consult the solubility table).
a3) Instead of sodium carbonate (Na2CO3) as the source of carbonate (CO32-) ions, what other carbonate compound could have been used? (consult the solubility table)
b. Lab 9 Experiment 2: Drinking Water Treatment: Removal of copper ions
b1) What did your precipitate of copper(II) carbonate look like?
Attach a photo of your filter setup for trapping copper(II) carbonate.
b2) Instead of copper(II) sulfate (CuSO4) as the source of copper ions to make copper(II) carbonate, what other copper compound could have been used as a source of copper ions? (consult the solubility table).
b3) Instead of sodium carbonate (Na2CO3) as the source of carbonate (CO32-) ions, what other carbonate compound could have been used? (consult the solubility table)
b4) Go ahead and add a little more sodium carbonate solution to this liquid that passed through the filter. Did more blue-green precipitate appear?
c. Lab 9 Experiment 2: Silver Salts for Photography
Attach a photo of your silver halide precipitates.
c1) What color would you call the silver chloride precipitate?
c2) What color would you call the silver bromide precipitate?
c3) What color would you call the silver iodide precipitate?
c4) Instead of sodium chloride (NaCl) as a source of chloride ions to make silver chloride, what other chloride compound could have been used as a source of chloride ions? (consult the solubility table).
c5) Instead of potassium bromide (KBr) as the source of bromide (Br-) ions to make silver bromide, what other bromide compound could have been used? (consult the solubility table)
c6) Instead of potassium iodide (KI) as the source of iodide (I-) ions to make silver iodide, what other iodide compound could have been used? (consult the solubility table)
d. Lab 9 Experiment 4: Double-Replacement reaction because of unstable product
d1) Instead of hydrochloric acid (HCl) as a source of hydrogen ions (H+)to make carbonic acid (H2CO3), what other acid could have been used as a source of hydrogen ions? (consult the solubility table).