A pure element is represented by the letter "A". The element represented by "A" attacks BC and replaces (displaces) the "B" element to make "AC".
A + BC → B + AC
Mg(s) + 2AgNO3(aq) → 2Ag(s) + Mg(NO3)2(aq)
Mg(s) + 2Ag+(aq) + 2NO3-(aq) → 2Ag(s) + Mg2+(aq) + 2NO3-(aq)
The fact that one metal can cause another metal to become undissolved is used in many applications.
1. Carry out single replacement reactions.
A. React metals with dilute acid to produce hydrogen gas.
B. React metals with salts of other metals to cause a single replacement reaction.
C. Use single replacement reactions to create voltage (make a battery).
2. List metals in order of activity based on your observation.
3. Write balanced chemical equations for single replacement reactions.

Sacrificial metal:
An example of a single replacement reaction is when one metal is sacrificed to save another metal. For example, concrete pillars have iron rebar in them for strength. However, salt water can quickly react with the iron to form iron (II) chloride. To prevent this, a metal like zinc or magnesium is attached to the rebar and will protect the iron. Here's the reaction:
Zn + FeCl2 → Fe + ZnCl2
Notice the zinc metal becomes zinc chloride and the iron that had reacted with the chlorine becomes metallic iron. This preserves the iron rebar. In a similar manner the aluminum in boats are preserved by a piece of zinc or magnesium. See the person pointing to a plug of magnesium that is in embedded in the boat's rudder? That keeps the corrosive salt water from dissolving the aluminum; the magnesium dissolves in aluminum's place.

Sacrificial metal:
Most hot water heater tanks are made from steel (iron), which can corrode in the presence of oxygen and water. Here is the reaction.
2Fe + O2 + H2O → 2Fe(OH)2
Each iron atom loses two electrons, which forms iron (II) hydroxide. A more reactive metal, such as magnesium (or zinc) can sacrifice itself by giving iron its electrons and forming magnesium hydroxide (magnesium corrodes instead of iron). So the single replacement reaction looks like this:
Fe(OH)2 + Mg → Mg(OH)2 + Fe
Magnesium replaces the iron so that iron can return to metallic iron.


Electrochemical Cells and Batteries:
The fact that different metals have a different pull on electrons can be exploited in the making of batteries (A battery is 2 or more electrochemical cells connected). A battery used in the 1800's for powering the telegraph was the "crow's foot" battery (because the zinc portion looked like a crow's foot). It used zinc and copper. It worked because copper ions have a stronger pull on electrons than does zinc. So electrons would leave the zinc, power the telegraph, and end up going to the dissolved copper. The "crows foot" shaped zinc would eventually dissolve as copper in solution would turn to copper metal. Here are the reactions. The first two are called half reactions and show what gives electrons (reducer) and what accepts electrons (oxidizer).
Zn(s) → Zn2+(aq) + 2 electrons (giving electrons)
Cu2+(aq) + 2 electrons → Cu(s) (takes electrons)
Zn(s) + Cu2+(aq) → Cu(s) + Zn2+(aq) (both giving and taking going on)


Electrochemical Cells and Batteries:
Another single replacement reaction involves how some metals will give their electrons to acids (H+) which dissolves the metal and produces hydrogen gas. One interesting story was the use of 500 lbs of potatoes to make a battery.
Amos Latteier (inventor/speaker) set up the big potato battery in a back of a truck. The wire was hooked to a boom box. He drove around neighborhoods to show it off. Nails with zinc coating (galvanized nails) and the natural acid in potatoes were the source of the electrical power. The copper pieces in the potatoes provided places where the electrons from the zinc were passed on to the acid. The first two equations are the half reactions that track the electrons. The last one puts the equations together.
Zn(s) → Zn2+(aq) + 2 electrons (zinc gives electrons)
2 electrons + 2H+(aq) → H2(g) (hydrogen ions take electrons)
Zn(s) + 2H+(aq) → Zn2+(aq)+ H2(g)
The electrons that zinc gave up had to travel through the radio before they got to the acid. That's the electrical current that ran the radio.

Precious Metal Recovery:
There are large numbers of X-ray films used my the medical community. At some point these are no longer needed and need to be disposed of. Silver is the main light sensitive ingredient in these films. Recovery of the silver is a good business. One process is to use nitric acid to dissolve the silver that is trapped in the film. After the silver is dissolved by nitric acid, you will have silver nitrate (AgNO3). Remember in solution, AgNO3(aq) is actually Ag+(aq) and NO3-(aq)
3Ag(s) + 4HNO3(aq) → 3AgNO3(aq) + 2H2O(l) + NO(g)
A cheaper (and more reactive) metal like magnesium could then be used to replace the silver ion and cause the silver ion to turn to metallic silver which sinks to the bottom of the solution and can be easily recovered. This is the same single replacement reaction shown at the top of this lab page.
Mg(s) + 2Ag+(aq) + 2NO3-(aq) → 2Ag(s) + Mg2+(aq) + 2NO3-(aq)
Activity Series of Metals (if only metals are listed)
Activity Series of the Elements (if metals and non-metals are listed)
Reactivity Series (another name for activity series)
The below activity series matches the one on the right, except this one identifies the metals in the top left column as reducers. They reduce the positive charge of ions of other metals. The elements at the bottom right are called oxidizers because they take away electrons from elements in the left column. Oxygen normally takes away electrons from other elements, so anything that acts like oxygen is called an oxidizer.

Activity Series of Metals (Also called Reactivity Series) |
||
| Li (Lithium) | Li+ | These metals are so reactive they cause water molecules to convert to hydrogen gas (H2) and hydroxide ions (OH-). See example below. 2Li + 2HOH → Li+ + 2OH- + H2 Note: After these metals react with water by giving up their electrons, they become positive metal ions and they lose their reactive property. For example, lithium metal is very reactive, but the lithium ion (Li+) is quite inactive. |
| K (Potassium) | K+ | |
| Ba (Barium) | Ba2+ | |
| Ca (Calcium) | Ca2+ | |
| Na (Sodium) | Na+ | |
| Mg (Magnesium) | Mg2+ | These metals are not reactive enough to cause water to convert to hydrogen gas and hydroxide ions unless the water is steam or very hot, then then reaction does take place. However, these metals are reactive enough to convert H+ ions into hydrogen gas (H2). Note, the metal ions (such as Zn2+) do not react with water or H+ ions. Only the pure metal reacts. |
| Al (Aluminum) | Al3+ | |
| Mn (Manganese) | Mn2+ | |
| Zn (Zinc) | Zn2+ | |
| Cr (Chromium) | Cr3+ | |
| Fe (Iron) | Fe2+ | |
| Cd (Cadmium) | Cd2+ | These metals are less reactive and will not react with water or steam, but will react with H+ ions by turning them into hydrogen gas (H2). |
| Co (Cobalt) | Co2+ | |
| Ni (Nickel) | Ni2+ | |
| Sn (Tin) | Sn2+ | |
| Pb (Lead) | Pb4+ | |
| H2 (Hydrogen) | H+ | Hydrogen gas is the reference element which the other elements are compared to. |
| Cu (Copper) | Cu2+ | These metals are the least reactive with respect to water or H+ ions. They will not react with water or convert H+ ions to hydrogen gas (H2). That makes these metals more inert, less likely to tarnish, and more valuable. These metals ions do not react with H+ ions, but they will react with hydrogen gas causing the hydrogen gas to become H+ ions. At the same time, the metal ions will become solid metal. See example. 2Ag+(aq) + H2(g) → Ag(s) + 2H+(aq) This is the reverse reaction that the metals above the H2 row is doing. In this reverse reaction the metal ion is taking away electrons and is the oxidizer. |
| Ag (Silver) | Ag+ | |
| Hg (Mercury) | Hg2+ | |
| Pt (Platinum) | Pt2+ | |
| Au (Gold) | Au3+ | |
| 2O2- (Oxide ion) | O2 |
On some Activity Charts, non-metals are included. Fluorine and oxygen are known to be the strongest oxidizers because they rip electrons off of other elements. After they take the electrons, they become ions (left column) which are quite inactive. |
| 2F- (Fluoride ion) | F2 |
|

They way to use this chart is to realize that any element in the left column will give its outer electrons (also called valence electrons) to any element in the right column that is below it. For example, magnesium metal will give its two electrons to any element in the right column below it starting with the aluminum ion (Al3+). In the example shown, magnesium gives up its two electrons to two H+ ions. In the process of doing that, magnesium metal becomes a magnesium ion (Mg→Mg2+), and at the same time the two H+ ions become hydrogen gas (H2←2H+). By the way, you will be doing this later as an experiment.

Combined it looks like the reaction below.
Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g)
Note: In the above reaction, magnesium metal is the reducer because it reduces the positive charge of the hydrogen ion (H+) as it becomes hydrogen gas (H2), which has no charge. Also, in the above reaction, H+ is considered an oxidizer, because it is taking away electrons from the magnesium metal. In all of these reactions, one is the oxidizer and the other is the reducer. It all depends on who is giving and who is receiving the electrons.
As another example, this chart shows that mercury metal will give its two valence (outer) electrons to oxygen gas. In the process, mercury becomes the mercury ion (Hg2+) and oxygen gas becomes the oxide ion (O2-). The mercury ion and oxide ions then bond together for form solid mercury(II) oxide, HgO.
2Hg(l) + O2(g)→ 2HgO(s)

In the above application of recovering silver from x-ray film, we said a cheaper metal could be used to cause the silver ions to turn to metallic silver. We also said that magnesium would work. If you look at the chart, magnesium is higher on the chart than the silver. That means magnesium is more active in giving its electrons away compared to silver. So magnesium will work. According to the chart any metal above silver starting with copper will give its electrons to the silver ion (Ag+). Even hydrogen gas would work. Lead and iron would probably be the cheapest metals to use.
Mg(s) + 2Ag+(aq) + 2NO3-(aq) → 2Ag(s) + Mg2+(aq) + 2NO3-(aq)
As you do this lab's experiments, the principles in using the activity series chart will be explained again.

Again, the more reactive elements (mostly metals here) will give their electrons to the less reactive metal ions. Note, that magnesium is higher than zinc, but magnesium will not give its electrons to zinc, only to zinc ions. Zinc metal atoms can't accept any electrons.
Also note that the positive metal ions in the right column can't give any electrons. They have already lost their electrons. So passing along of electrons starts in the left column and is handed to anything listed in the right column that is below the element in the left column.
All elements can be a reducer if it has an element below it in the chart. In other words, it can reduce the positive charge on the element below it. The most active reducers are the ones at the top of the chart in the left column. Lithium being the strongest.
Anything in the right column can be an oxidizer if it has an element above it. It oxidizes the element in the left column above it by pulling off and accepting the other elements electrons.
The elements at the bottom of the right column are the most reactive oxidizers. Fluorine gas is the strongest listed. If you put the strongest oxidizer (F2) with the strongest reducer (lithium metal), the reaction will be violent.

When there is a tendency for one thing to push its electrons over to something else that wants those electrons, then there is an electrical voltage. Sometimes the relative activity of these metals is listed with the voltage that they would generate if in contact with H+ ions. Hydrogen is set as zero volts. Lithium has the highest negative voltage because it will build up an the highest excess of electrons as it tries to give them to hydrogen ions.
So a measurement of voltage is giving an indication that electrons are wanting to travel from a metal to the ions of a different metal. Again, a voltage will only occur if the metal is in a row higher than the metal ion. It doesn't always have to be a metal ion. At the bottom of the chart are some non-metals. On one row, the oxidizer (the one that grabs electrons) can even be a combination, such as the dichromate ion (Cr2O72-) with hydrogen ions (H+). That combination is better at ripping electrons off of other elements than either one by itself.
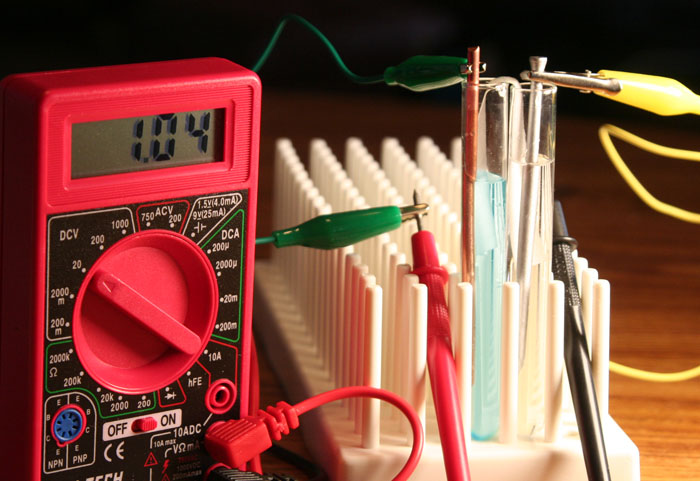
In this lab, you will be using a multimeter set to measure voltage to measure this voltage that these metals and ions can create.

Activity (Reactivity) of Magnesium Metal with Dilute Acid:
According to the activities chart, all metals above hydrogen will produce hydrogen gas if in an acidic solution (H+ ions are present). One of those metals that sit above hydrogen is magnesium. Your kit has two strips of magnesium ribbon. Take one out and break off 5 quarter inch pieces. You will be using them in the experiments below.

Magnesium is several steps up from where hydrogen is. So it should be rather reactive when in contact with H+ ions in the 0.1 M HCl (hydrochloric acid) solution in your kit. As magnesium reacts, if should dissolve.
Mg(s) + 2H+(aq) + 2Cl-(aq) → Mg2+(aq) + H2(g) + 2Cl-(aq)
Notice, the chloride ions (2Cl-) don't get involved in this reaction. So they don't need to be included in these reactions. So we can rewrite it more simply:
Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g)
As you can see, magnesium gives its electrons to the H+ ions. With two missing electrons, magnesium is now +2 charge. Hydrogen ion (H+) gaining an electron will become neutral. It will then pair up with another neutral hydrogen atom to make H2 gas.

Place one of the small (1/4 inch) pieces of magnesium ribbon into a clean test tube. The add about 10 drops of 0.1 M HCl.
You will be looking for bubbles. Those, according to our chemical equation, should be hydrogen.
Mg(s) + 2H+(aq) → Mg2+(aq) + H2(g)
Another evidence of hydrogen forming is that the magnesium strip will be floating rather than sinking to the bottom of the liquid. The bubbles make it lighter so it will float.

You should also look at the reaction using the mini-microscope in your kit.
Under the microscope you get a better feel for all the activity that happens when magnesium is attacked by hydrochloric acid.
a1) What is your description of what you see through the microscope as the magnesium is being dissolved by hydrochloric acid?
Note: If the water in this solution is allowed to evaporate, magnesium chloride (MgCl2) will be the salt that remains.
Activity (Reactivity) of Aluminum Metal with Dilute Acid:
We are going to try aluminum next. According to the activity chart, aluminum should also give its outer electrons to the acid (H+ ions) because aluminum metal sits above the H+ ions.
Your kit has some small sheets of aluminum in the plastic case with the candles. Take out a sheet and tear off about 1/4 of it and crumple it up.

Place the crumpled up aluminum in another clean test tube. Add about enough drops of 0.1 M HCl to cover it with acid.
Look closely for any sign of bubbles.

Use the mini-microscope to see if you see any bubbles streaming from the aluminum. If you see bubbles, the below reaction is happening:
2Al(s) + 6H+(aq)→ 2Al3+(aq) + 3H2(g)
a2) Did you see any bubbles from from the aluminum foil in the 0.1 M hydrochloric acid solution?

According to the activity chart, aluminum should give its 3 outer electrons to the H+ ions. The H+ ions should turn into hydrogen gas. However, you may not see this behavior. Why? The answer comes from the previous lab (Lab 7). It talked about the toughness of metal oxides and aluminum oxide was mentioned specifically. The surface of all aluminum items is covered with a layer of aluminum oxide (Al2O3) because it reacts with the oxygen in the air. The aluminum oxide makes it quite resistant to corrosion.
It one could sand a piece of aluminum and quickly add acid, then hydrogen gas will form. However, it's not easy sanding aluminum foil.
We did place a few drops of acid on a piece of aluminum foil and then scratched the foil under the acid with a glass rod. Using the microscope we could see small bubbles forming where those scratches are. So aluminum does react, it's just not easy getting through the aluminum oxide coating.

Activity (Reactivity) of Zinc Metal with Dilute Acid:
If we look at the Activity series above, we see that zinc also sits above hydrogen. So zinc should react with acids as well.
Your kit has a small plastic case with 2 alligator leads, two pieces of copper wire, and two iron nails that are coated with zinc. They call these galvanized nails. The zinc protects the iron from corrosion (rusting). Below is the reaction of acid with zinc. Zinc will dissolve and hydrogen bubbles will be produced.
Zn(s) + 2H+(aq)→ Zn2+(aq) + H2(g)

Fill an clean empty test tube about 1 inch deep with 0.1 M HCl solution. Clip one end of one of the alligator clip leads to the top of the nail. This will help you pull the nail out of the test tube. Lower the nail carefully into the test tube.
Look for bubbles coming from the nail.
They may be hard to see because zinc also forms a protective cover on its surface with zinc oxide.

Use your mini-microscope to look for bubbles on the nail. Tilt the nail so it is sitting right next to the glass, allowing the microscope to focus on it. Look at the very tip of the nail.
a3) Do you see any bubbles coming from the zinc-coated nail when it was submerged in the 0.1 M HCl acid solution?
Note: If the water in this solution is allowed to evaporate, zinc chloride (ZnCl2) will be the salt that remains.

Activity (Reactivity) of Copper Metal with Dilute Acid:
Now we will check to see if copper metal will react with acid. You should have already found a length of heavy copper wire.
Find a clean empty test tube and fill it to about 1 inch with 0.1 M HCl solution (see below image).

The copper wire is shorter than the test tube, so you can use the alligator clip to hold it.
Look for any bubbles streaming from the copper wire.
a4) Did you see any bubbles streaming from the copper wire as it was in contact with 0.1 M hydrochloric acid?

Here is the part of the activity series showing hydrogen and copper. We see copper below hydrogen not above it like the other metals we tested. So according to the chart, copper is less active (less reactive) than hydrogen ions. So it should not give any of its electrons to the H+ ions. So there will be no reaction by placing copper metal in contact with an acid like H+.
Cu(s) + 2H+(aq)→ No Reaction

For this experiment you will be using these four chemicals plus the small 1/4 inch pieces of magnesium.
You will also need four clean test tubes. So you may need to rinse out the test tube used in experiment 1 above. First rinse with tap water. You may need to use the test tube brush to clean them also. After a rinse with tap water, rinse them with purified water (distilled water). It's OK if there is still water in the test tubes. You don't have to wait for them to dry.

Magnesium metal and Copper Ion Single Replacement:
According to the Activity Series Chart, magnesium is above copper, so it should give its electrons to the copper(II) ion. When it does, the magnesium should dissolve and copper ions should become particles of copper metal.
Mg(s) + Cu2+(aq)→ Mg2+(aq) + Cu(s)
Note: It doesn't work the other way around. Magnesium ions will not react with copper metal because magnesium is above copper in the activity series.
Mg2+(aq) + Cu(s) → No Reaction

Your kit has some copper(II) sulfate crystals. Take a crystal out and put it into a clean test tube. Add purified water to a depth of about 1 inch. When copper(II) sulfate dissolves in water, we get these ions:
CuSO4(s) + H2O → Cu2+(aq) + SO42-(aq)
Let the crystal sit. It takes about 10 minutes for it to dissolve. Meanwhile you can do the next reaction.
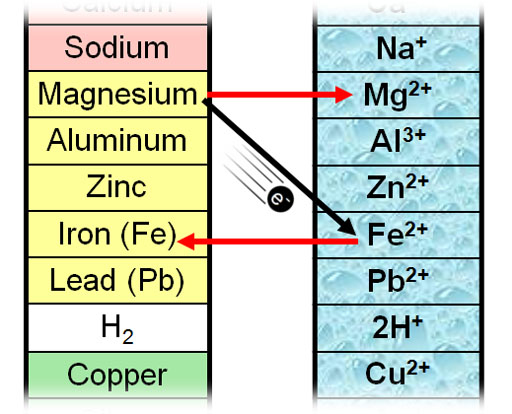
Magnesium metal and Iron Ion Single Replacement:
Here is the portion of the Activity Series showing magnesium sitting above iron. Because of that, magnesium should give its electrons to iron(II) ions. When it does, magnesium will dissolve and become Mg2+ and iron ions should become particles of iron metal.
As a source of Fe2+ ions, we will use iron(II) sulfate. Below is the single replacement reaction.
Mg(s) + FeSO4(aq) → Fe(s) + MgSO4(aq)
You can see that magnesium (Mg) replaces Fe in the FeSO4 compound above. That makes it a single replacement reaction. The full ionic equation is below. In water the FeSO4 becomes separate ions (Fe2+ and SO42-). The below equation also shows that magnesium dissolves as Mg2+.
Mg(s) + Fe2+(aq) + SO42-(aq) → Fe(s) +Mg2+(aq)+ SO42-(aq)

Take a scoop of FeSO4 from its test tube and place in an clean empty test tube. Add purified water (about 2 mL, which is about 1 inch deep). Put a cap on the test tube and shake it to get the powder to dissolve.
The solution will be yellowish, but you will find that it doesn't all dissolve. There are particles settled at the bottom of the test tube.

Use the microscope to examine these particles that don't dissolve. Apparently, iron(II) sulfate exposed to oxygen in the air will decompose into iron(III) sulfate and iron(III) oxide. That probably accounts for these particles.
This is a good lesson to learn. Even though a product has a label on it, it doesn't guarantee that it only contains that one ingredient.
b1) In the iron(II) sulfate solution, describe the particles you see through the mini-microscope.

Here are some of the particles we saw under the microscope. The large yellow particles are likely iron(III) sulfate, which can be yellow or even a bluish white. The small white particles may also be iron(III) sulfate. Different crystal structures of the same formula can have different colors.
One black particle is near the bottom right corner of the image. That's probably iron(III) oxide with formula of Fe2O3.
When we add the piece of magnesium to the solution, we don't want it to react with these particles, just the Fe2+ in the solution. So we want to filter out these particles. You will make a mini-funnel/filter like you did in a previous lab.


It is also helpful to cut the stem of the disposable pipette in half. That allows it to sit all the way down into a test tube.
Last time for filter paper we used a piece of paper towel. This time we want something that will do a little better job.
Locate the ziploc bag with filter paper in the back of your kit (behind the back panel).





Now transfer the iron(II) sulfate solution to the small paper filter. The mini-funnel/filter will capture the particles that are contaminating the solution.
Take a photo of your setup with the mini-funnel/filter.

After the solution is filtered, it will be a yellow color, but there won't any of those particles contaminating the solution.
In Lab 6, the table that showed colors for different elements said that Fe2+ [iron(II)] was a greenish color and Fe3+ [iron(III)] was a yellow color. The yellow color of our dissolved iron(II) sulfate indicates that there is some iron(III) sulfate in the solution. As far as magnesium is concerned, it will give electrons to either Fe2+ or Fe3+. So it doesn't affect the experiment we are doing here.

Drop one of the small pieces of magnesium ribbon into the filtered iron sulfate solution. Start making observations.
You should notice that it starts turning black rather quickly. That is iron ions that are turning into small particles of iron metal. Take a look at it using your mini-microscope. The reaction is much more impressive through the microscope.
b2) In the iron(II) solution with the magnesium ribbon, describe what you see through the mini-microscope.
An unexpected reaction is the production of bubbles. There could be two possibilities. One possibility is the energy of the single replacement reaction of magnesium replacing iron ions is causing magnesium to react with water to form hydrogen gas. The other possibility is that the solution is somewhat acidic. Remember, in the first experiment of this lab, you added acid to magnesium and it created hydrogen gas.

To see if the solution is acidic, get one of the pH test strip papers from the 0-14 pH test strip test tube. Use the same disposable pipette that you used to make the transfer of unfiltered iron sulfate solution to place a few drops of the solution onto a pH test strip. When we did it, we saw that the solution had a pH between 3 and 4. Again, some contamination in the iron(II) sulfate powder has created a weakly acidic solution. So that could contribute to the magnesium creating hydrogen bubbles.
b3) What pH did you read on your pH test strip?
2Mg(s) + Fe2+(aq) + SO42-(aq) + 2H+(aq) → Fe(s) + 2Mg2+(aq)+ SO42-(aq)+ H2(g)
The sulfate ion is not involved, so we could simplify the above reaction showing only magnesium, iron, and hydrogen: Again, magnesium is donating electrons to both iron ions and hydrogen ions.
2Mg(s) + Fe2+(aq) + 2H+(aq) → Fe(s) + 2Mg2+(aq)+ H2(g)

Magnesium metal and Copper Ion Single Replacement:
Earlier we had you put a crystal of copper(II) sulfate into some purified water. It should be dissolved by now.
Take one of the short pieces of magnesium ribbon and drop it into the copper sulfate solution.

Magnesium metal and Copper Ion Single Replacement:
According to the Activity Series Chart, magnesium is above copper, so it should give its electrons to the copper(II) ion. When it does, the magnesium should dissolve and copper ions should become particles of copper metal.
Mg(s) + Cu2+(aq)→ Mg2+(aq) + Cu(s)
Note: It doesn't work the other way around. Magnesium ions will not react with copper metal because magnesium is above copper in the activity series.
Mg2+(aq) + Cu(s) → No Reaction

The reaction of magnesium with copper ions is rather interesting. Be sure to look at it through the microscope. Again, we get some unexpected bubbles. We checked the pH of the copper sulfate solution and it was essentially neutral. So it seems the extra energy released from the single replacement reaction causes some of the magnesium to react with water to form hydrogen gas.
The eye mostly sees small black particles falling from the magnesium strip. These are small particles of copper metal. However, through the microscope many other colors and particles are visible.
b4) Describe what you see in as the magnesium reacts with the copper(II) sulfate solution as you look through the mini-microscope.

Here is what we saw in the microscope. At first the black spongy material had lavender colored ends to it. After a while, the lavender colored looked like copper metal. We also see some yellow-green particles. These are probably some other copper compounds. The air has oxygen and carbon dioxide in it. So these might be copper oxides and copper carbonate compounds that formed during this reaction. The large white flakes are unknown.
Purity is always a concern to chemists. They know that very few things are pure. When buying chemicals, there are different grades of purity. For example, "Technical grade" is not as pure as "Reagent Grade". In our experiment here, the purity of the magnesium and the copper(II) sulfate may not be very high. This could also explain that side reactions are producing some of the other colored particles.

Magnesium metal and Potassium Ion Single Replacement:
Your kit has some potassium bromide (KBr). Place a small amount in a clean test tube and dissolve it with purified water. Use another small piece off of the magnesium ribbon and place it in the KBr solution. Here is the equation:
Mg(s) + 2K+(aq) + 2Br-(aq) →? Mg2+(aq) + 2K(s) + 2Br-(aq)
The question is do you expect this reaction to occur? In other words, will magnesium metal give its electrons to potassium ions, which will cause magnesium to dissolve and potassium metal particles to form?
What do you see when you mix the two, if anything?
Below is the Activity Chart showing potassium and magnesium.

b5) Will magnesium metal give its electrons to potassium ions (K+)?
Here is a follow-up question.
b6) Will potassium metal give its electrons to magnesium ions (Mg2+)?

Magnesium metal and Silver Ion Single Replacement:
We have twice mentioned the example of recovering silver from x-ray film. We mentioned that one way this is done is by dissolving the silver in the x-ray film with nitric acid, which will create a solution of silver nitrate. Then we mentioned that magnesium metal could cause the silver ions in the solution to become particle of silver metal. Your kit has some silver nitrate solution. So you can test the claim that magnesium will cause the silver to come out of solution. Below is the reaction.
Mg(s) + 2Ag+(aq) + 2NO3-(aq) → 2Ag(s) + Mg2+(aq) + 2NO3-(aq)
The nitrate ion does not participate in this reaction so we can remove it. (FYI: Ions that do not participate are called spectator ions).
Mg(s) + 2Ag+(aq) → 2Ag(s) + Mg2+(aq)
When the silver ions turn into microscopic particles of silver metal, they won't look like the silver in a silver coin. They usually look black or gray.


You should see a quick reaction of the magnesium metal reacting with silver ions. Use your microscope to get a close-up view.
b7) How would you describe what you see in the microscope as the silver ions react with the magnesium metal?

The photo doesn't show it very well, but to us, the silver metal that was forming appeared to be a dark gray spongy material. After a while, some areas were light gray and some almost white.
Again, this form of silver would need to be melted down in order to get the silvery metal look.

The Two-Compartment Electrochemical Cell:
In the above single replacement reactions, the more active metal gave its electrons directly to the ion of a different metal. The electrochemical cell does the same thing but the electrons must pass through a wire first. This allows us to develop voltage and power some electrical device. The two compartments (two beakers) separate the active metal from the ions that it will be sending its electrons.
Earlier you added magnesium ribbon to a copper(II) sulfate solution. The magnesium atoms gave its electrons directly to the Cu2+ ions. In the setup on the left, the metal magnesium atoms send their electrons through the wire, through the voltmeter, through the copper strip, and finally to the Cu2+ ions in the copper sulfate solution on the right. Copper ions become copper metal and the magnesium atoms that sent their electrons become magnesium ions. So the overall equation is the same as before. The main difference is that magnesium metal and copper ions were never in direct contact with each other.:
Mg(s) + Cu2+(aq)→ Mg2+(aq) + Cu(s)

To measure voltage, you will need to set up your multimeter to measure voltage. Locate the meter and find the small clear plastic case that has the black and red test leads for the multimeter.
1) locate the small arrow on the dial.
2) Move the arrow so it points to 20. This means it can measure up to 20 volts of direct current (DC) electricity. The "DCV" letters stand for direct current voltage. This is voltage that causes electricity to flow in one direction. This is like the batteries in your car, cellphone, or whatever. They have a negative terminal and a positive terminal. The electrons only flow from the negative terminal to the positive terminal. That's called direct current.
3) Connect the black test lead plug to the input jack that sits next to the label "COM". "COM" is short for common ground. This is the negative input. In other words, this is where it expects an excess of electrons, which will make it negatively charged.
4) Connect the red test lead plug to the jack that sits next to the label "VΩmA". The "V" stands for volts. The Ω (ohm symbol) is for measuring electrical resistance, and "mA" means milliamps, which measures small electrical currents. Since our dial is pointing to 20 volts DC, then we will be measuring voltage "V".
5) Notice the on-off switch. When ready, you will switch it on.
(3 & 4 ) Make sure the red and black test lead plugs are pushed in all the way.

In this experiment, you will be using copper(II) sulfate again. Like mentioned before, copper(II) sulfate dissolves slowly. So for now take a couple of crystals out and put them into a clean test tube. Add purified water to fill the test tube about 1/2 full.
Let the crystals sit. It takes about several minutes for it to dissolve. Meanwhile you can do the next experiment.

In experiment 1, zinc (on a galvanized nail) gave its electrons directly to hydrogen ions (0.1 M HCl solution). In doing that zinc would dissolve and hydrogen ions formed hydrogen gas bubbles. You may remember seeing bubbles coming from the zinc coated nail through the microscope.
If set up correctly, zinc can pass its electrons through a wire to get its electrons to the hydrogen ions.
Zn(s) → Zn2+(aq) + 2 electrons
Zinc atoms will dissolve and give up 2 electrons which go into a wire.
The 2 electrons from the wire react with a hydrogen ions (H+) to form hydrogen gas. So we get the reaction as before but the zinc is not directly in contact with the hydrogen ions.
Zn(s) + 2H+(aq)→ Zn2+(aq) + H2(g)
So its a rather neat trick that they can react with each other over a distance.