
The word Nomenclature comes from Nomenclator, who is a person who calls out names. "Nomen" means names and "clator" is a person who calls out. When students get their diploma, the nomenclator is the person who calls out the students' names. In chemistry, "nomenclature" is learning to call out chemical names. Understanding chemistry nomenclature is like learning any language. It takes practice and study, but the reward is that it gives you the ability to talk to people you couldn't talk to before.
Here is a link to a menu page to tutorials on nomenclature. These have the bulk of the information you need to learn chemical nomenclature. You ought to do those before you do this lab. In other words, there will be a minimum of instructions on nomenclature in this lab except for giving you many examples to use as a pattern. Also, this lab will test your knowledge of nomenclature.
http://www.chemistryland.com/CHM130S/06-Nomenclature/NomenclatureMenu.htm

Learning nomenclature is mostly a mental exercise. So this lab also has some hands-on activities to investigate some of the chemicals that you are learning to name.
Qualitative Inorganic Analysis is the identification of certain inorganic ions (both positive and negative). "Qualitative" means you are saying if the substance is present, but you aren't saying how much is present. Quantitative analysis is the analysis that comes up with how much is present. In this lab, you will be doing both qualitative and quantitative analysis of inorganic compounds.
Inorganic compounds often contain metals bonded with non-metals. The are often called "salts" or "metallic salts". Inorganic compounds are also non-metals bonded with non-metals. Organic compounds, as your remember, are based on carbon and chains of carbon which are normally just bonded to other non-metal elements like hydrogen, oxygen, and chlorine.
Nomenclature Objectives
1. Diatomic elements. Learn the seven diatomic elements. (Examples: O2, N2, Cl2)
2. Monatomic ions. Learn the correct charges of monatomic ions. (Examples: Na+, Ca2+, Cl-, Br-)
3. Binary Compounds (compounds made from 2 different elements)
3a. Binary Compounds with ionic bonding: Write names and formulas of binary compounds that use ionic bonding. These are positive metal ions that have bonded with negative non-metal ions. (Examples: NaCl-sodium chloride, CaBr-calcium bromide, FeCl3-Iron(III) chloride).
3b. Binary Compounds with covalent bonding: Write names and formulas for binary compounds that use covalent bonding. These are non-metals that have bonded with other non-metals by sharing their electrons (covalent bonding). (Examples: CO2-carbon dioxide, SO3-sulfur trioxide, CCl4-carbon tetrachloride).
4. Polyatomic ions. Learn names, formulas, and correct charges of polyatomic ions. (Examples: SO42- is called sulfate. It has a -2 charge.)
5. Ternary Compounds. Learn names and formula of ternary compounds (compounds with 3 different elements).
Inorganic Analysis Objectives
6. Use the color of a salt to help identify certain metal ions.
7. Use a flame color test to help identify certain metal ions.
8. Use chemical test strips to help identify various ions and determine their concentration.
Learning nomenclature can be like learning words to another language, but it doesn't help if you learn the word and don't know what it is. For example, you can learn how to pronounce and write the French word, "la pomme". That is fine, but you also need to know what it means (It means apple by the way). The same is true in chemistry nomenclature. This lab has a lot of chemical names that you will learn to write. But this is just part of learning about chemicals. You should also know what they look like and what they might be used for. That's when Wikipedia or Google is useful. Just do a search in Wikipedia and you often get a picture plus some background information on that chemical compound.

"Diatomic" means it contains two atoms of the same element ("di" means two). Some elements are very unstable (reactive) if they are moving around as a single atom. Therefore the single atom often combines with another single atom (of the same element) to make a much more stable diatomic molecule. Oxygen is one example. That's why it's often referred in medical circles as "O-two" because its formula is O2. The following are the 7 diatomic elements:
H2, O2, N2, F2, Cl2, Br2, I2. The names in the same order are: hydrogen, oxygen, nitrogen, fluorine, chlorine, bromine, and iodine. Even though there are 2 atoms in each of these, the proper names do not indicate two atoms. For example, H2 is called hydrogen not dihydrogen. Even though medical staff may call O2 (O-two), chemists simply say "oxygen".
In the situation where a single atom of one of these element is not paired with itself, the name is different. For example, if there is just a single oxygen atom produced that hasn't yet paired with another single oxygen atom, those single oxygen atoms are called oxygen radicals or oxygen free radicals. In an equation, they are usually indicated by a dot. For example, when ultraviolet light splits an O2 molecule, the equation is written as:
O2 → O• + O• . In organic chemistry, chlorine gas is often exposed to UV light to get Cl2 → Cl• + Cl• . These free radicals are much more reactive than when they were paired. The chlorine radical (Cl•) has the ability to attach itself to an organic compound, which is useful making new compounds.
The word "monatomic" is a shortening of mono & atomic. The word monatomic would also indicate that these normally diatomic elements are moving about as a single atom. So monatomic fluorine means an atom of fluorine moving about as a single atom. Where as "fluorine" means it is moving about as a pair of fluorine atoms.

When you learn a language, one of the first things you learn is how to count. In chemistry, we use Greek to count.
1= mono (mono isn't used much because the count of 1 is understood)
2 = di
3 = tri
4 = tetra
5 = penta
6 = hexa
7 = hepta
8 = octa
9 = nona
10 = deca
The below table shows how the non-metals on the left will combine with the non-metals across the top. You should see the pattern. The number of the atoms in the formula is indicated with the appropriate Greek prefix shown above. The last element named has its name shortened and the suffix "ide" added to it. For example, chlorine becomes chloride. The "ide" ending is the signal that you are dealing with a binary compound (a compound with just two elements). Check your understanding by answering what a1, a2, etc. are in the below table.
| Fluorine | Chlorine | Oxygen | Sulfur | |
| Carbon | CF4-Carbon tetrafluoride | CCl4-Carbon tetrachloride | CO2-Carbon dioxide | CS2-Carbon disulfide |
| Nitrogen | NF3-Nitrogen trifluoride | NCl3-Nitrogen trichloride | N2O-dinitrogen oxide | N2S3-dinitrogen trisulfide |
| Hydrogen | HF-Hydrogen fluoride | HCl-hydrogen chloride | H2O-dihydrogen oxide* | H2S-dihydrogen sulfide |
| Silicon | SiF4-Silicon tetrafluoride | SiCl4-(a1) | SiO2-(a2) | SiS2-(a3) |
| Phosphorus | (a4)-Phosphorus trifluoride | PCl3-(a5) | (a6)-triphosphorus pentoxide | P2S3-(a7) |
*Note: Even though the rules give H2O the name of dihydrogen oxide (or dihydrogen monoxide), chemists still call this compound simply water.
The other type is when a metal combines with a non-metal. Let's begin with the one metal cation (cation means a positive ion) combining with a non-metal anion (anion means a negative ion).
Tip on finding formula: Atoms that have a charge have a one-track mind. They are attracted to anything with the opposite charge. In the table below the positive metal ions on the left column will all be attracted to the negative non-metal ions on the top row. If the positive metal ion is +1 and the negative ion is -1, after they combine, their charges balance out to zero. Being zero charge, they will not attract any other atoms. So the final formula is set as one each. For example, Na+ + Cl- → NaCl. Also, Ag+ + Cl- → AgCl. If the metal ion is shows "2+" and the non-metal ion shows "2-", they will also cancel their charges when they combine. So the final formula is one each. For example, Fe2+ + O2- → FeO. Now lets look at Na+ being attracted to O2-. After they combine, there's still a negative one charge because (+1) + (-2) = (-1). So NaO still has a negative one charge (NaO-). That means it will attract another positive sodium ion (Na+). So, NaO- + Na+ → Na2O. At this point the charges all cancel and Na2O is neutral so there's no more attraction, meaning that's the final formula. Remember, atoms can't think, but they can still make the right formula by simply coming together as long as there's a charge present.
Tip on finding name for a metal combining with a non-metal: When there are just two kinds of atoms (a binary compound), the name of the compound begins with the name of the positive metal ion. The second word is the name of the negative non-metal ion, except the name is shortened and "ide" is added. Most of these are done so you can learn the pattern. Others are blank for you to do. The blanks ones will be shown in the lab summary as the reaction. For example, (b1) will be shown as
b1)
Na+ + S2- → ? :
And (b2) will ask for the name of (b1).
Cl- |
O2- |
N3- |
S2- |
P3- |
|
Na+ |
NaCl Sodium chloride |
Na2O Sodium oxide |
Na3N Sodium nitride |
(b1) (b2) |
Na3P Sodium phosphide |
Mg2+ |
MgCl2 Magnesium chloride |
MgO Magnesium oxide |
Mg3N2 Magnesium nitride |
(b3) (b4) |
(b5) (b6) |
Al3+ |
(b7) (b8) |
Al3O2 Aluminum oxide |
AlN Aluminum nitride |
Al2S3 Aluminum sulfide |
AlP Aluminum phosphide |
Fe2+ |
FeCl2 Iron(II) chloride |
FeO Iron(II) oxide |
(b9) (b10) |
(b11) (b12) |
Fe3P2 Iron(II) phosphide |
Ag+ |
AgCl Silver chloride |
Ag2O Silver oxide |
Ag3N Silver nitride |
(b13) (b14) |
(b15) (b16) |
Fill in the missing information indicated by "b" followed by a number.
Name |
Formula Note: Some equations are given to show how the ions combined to make the final compound |
| Potassium sulfide | K+ + K+ + S2- → (b17) Note: The final compound has no charges on it. |
| Silver bromide | Ag+ + Br- → (b18) |
| Calcium chloride | Ca2+ + Cl- + Cl- → (b19) |
| Nickel(II) sulfide | Ni2+ + S2- →(b20) |
| Magnesium phosphide | Mg2+ + Mg2+ + Mg2+ + P3- + P3-→(b21) |
| Calcium oxide | (b22) |
| Iron(III) iodide | (b23) |
| (b24)* | Cu3P |
| *Since the phosphorus ion is a -3 charge and we see 3 copper atoms, that means the the copper must have a plus one charge. 3x(+1) balances with -3. Use a Roman numeral to indicate the charge on the copper atom. | |
| (b25)* | NaH |
| *You have normally seen hydrogen in front, such as HCl. That's because the hydrogen in HCl is positive. In NaH, the hydrogen is negative because sodium (Na) has a much greater tendency to give away its electron compared to hydrogen. When hydrogen is negative in this kind of compound, its name is hydride. | |
c. Ternary Compounds (made of three elements)
Ternary Compounds
CN- |
NO3- |
CO32- |
SO42- |
PO43- |
|
NH4+ |
NH4CN Ammonium cyanide |
NH4NO3 Ammonium nitrate |
(NH4)2CO3 Ammonium carbonate |
(c1) (c2) |
(NH4)3PO4 Ammonium phosphate |
Mg2+ |
Mg(CN)2 Magnesium cyanide |
Mg(NO3)2 Magnesium nitrate |
(c3) (c4) |
(c5) (c6) |
(c7) (c8) |
Al3+ |
Al(CN)3 Aluminum cyanide |
(c9) (c10) |
Al2(CO3)3 Aluminum carbonate |
Al2(SO4)3 Aluminum sulfate |
(c11) (c12) |
Cu2+ |
Cu(CN)2 Copper(II) cyanide |
Cu(NO3)2 Copper(II) nitrate |
CuCO3 Copper(II) carbonate |
(c13) (c14) |
Cu3(PO4)2 Copper(II) phosphate |
| Notice that iron(III) below has the same charge as aluminum above so the number of the anions (negative ions) will be the same. Since iron has more than one oxidation number, we must indicate which one it is. When aluminum combines, it only has a +3 charge, so there's no reason to indicate the charge with aluminum. | |||||
Fe3+ |
Fe(CN)3 Iron(III) cyanide |
(c15) (c16) |
Fe2(CO3)3 Iron(III) carbonate |
Fe2(SO4)3 Iron(III) sulfate |
(c17) (c18) |
| To find names, you can learn the rules. Also, if you have Internet access, try typing the formula in the search field (like in Google). For example, to find the name of H2CO3 type H2CO3 in the search field. You will likely see websites that show the name of carbonic acid. Also, you can type the name in a search engine and often find the formula. | |||||
H+ |
HCN hydrogen cyanide if gas hydrocyanic acid if in water |
(c19) (c20) |
H2CO3 Carbonic acid |
(c21) Sulfuric acid |
H3PO4 (c22) |
Name |
Formula (Note: An equation is given on some to show how the ions combined) |
| Potassium sulfate | K+ + K+ + SO42- → (c23) |
| Ammonium bromite | NH4+ + BrO2- → (c24) |
| Zinc nitrate | Zn2+ + NO3- + NO3- → (c25) |
| Nickel(II) sulfite | Ni2+ + SO32- → (c26) |
| Iron(II) carbonate | (c27) |
| Magnesium hydrogen phosphate | Mg2+ + HPO42- → (c28) Note: Phosphate (PO43-) has negative 3 charge, but when combined with H+, one of the negative charges is canceled. So the result is a negative 2 charge. Since Magnesium ion is plus 2, the charges combine to be zero if you have one magnesium ion and one hydrogen phosphate ion. |
| Calcium chlorate | Ca2+ + ClO3- + ClO3- → (c29) |
| Cobalt(III) acetate | Co3+ + C2H3O2- + C2H3O2- + C2H3O2- → (c30) |
Above, it shows what ions are used to make a particular salt (an ionic compound). However, it didn't show what produced those ions. These ions can come from an acid and a base. In this situation, the acid disassociates into H+ ions and a negative ion (anion). The base disassociates into OH- ions and a positive ion (cation). The H+ and OH- ions combine to form water, leaving the anion and cation to combine to make the salt (see table below).
"Parent acid" and "Parent base" mean that you can start with this pair of acid and base and they produce a salt. For example, starting with phosphoric acid, one can add the base called sodium hydroxide (NaOH), and it will form salt called sodium phosphate (Na3PO4). Here's the reaction in water (aq):
2H3PO4(aq) + 6NaOH(aq) → 2Na3PO4(aq) + 3H2O(l)
The below equation is the full ionic equation of the above equation. The full ionic equation shows the acid's anion (PO43-) and the base's cation (Na+). Those two ions form the salt.
6H+(aq) + 2PO43-(aq) + 6Na+ + 6OH-(aq) → 6Na+(aq) + 2PO43-(aq) + 3H2O(l)
The acid (H+) and the base (OH-) make water. If the solution is dried, Na+ and PO43- will combine to form the salt called sodium phosphate, which is Na3PO4(s). Notice the charges have to balance, so it takes 3 of the Na+ ions to balance the -3 charge of PO43-.
Here is reaction to of sulfuric acid reacting with copper(II) hydroxide to make copper(II) sulfate:
H2SO4(aq) + Cu(OH)2(aq) → CuSO4(aq) + 2H2O(l)
If dried, you get the blue salt, copper(II) sulfate, CuSO4.
The below table is partially filled out so you can learn the pattern for using an acid and base to make a salt. "d" followed by a number are the ones you answer in the lab report. The chart shows that a salt is produced. What is not shown is that water is also produced when these acids are mixed withe these bases. The salt is what remains after drying.
Parent Acid |
Parent Base |
Salt (produced) |
||||
| Acid Name | Acid Formula | Anion |
Base Formula |
Cation |
Formula |
Name |
| Phosphoric acid | H3PO4(aq) |
PO43- |
NaOH |
Na+ |
Na3PO4 | sodium phosphate |
| Hydrochloric acid | HCl(aq) |
Cl- |
NaOH |
Na+ |
NaCl | (d1) |
| Hydroiodic acid | HI(aq) |
I- |
KOH |
K+ |
(d2) | (d3) |
| Chromic acid | H2CrO4(aq) |
CrO42- |
(d4) |
K+ |
K2CrO4 | potassium chromate |
| Sulfuric acid | H2SO4(aq) |
SO42- |
Cu(OH)2 |
Cu2+ |
CuSO4 | copper(II) sulfate Since sulfate is -2, Cu must be +2. Copper has varied charge so we show (II) |
| Carbonic acid | H2CO3(aq) |
CO32- |
Ca(OH)2 |
Ca2+ |
CaCO3 | (d5) |
| (d6) | H2SO4(aq) |
SO42- |
(d7) |
Mg2+ |
MgSO4 | (d8) |
| Hydrochloric acid | HCl(aq) |
Cl- |
Co(OH)2 |
Co2+ |
(d9) | Cobalt(II) chloride |
| Sulfuric acid | (d10) |
(d11) |
Fe(OH)2 |
(d12) |
FeSO4 | (d13) *iron has multiple charges |
| Hydrochloric acid | (d14) |
(d15) |
(d16) |
Ni2+ |
NiCl2 | (d17) *nickel has multiple charges |
| Sulfuric acid | H2SO4(aq) |
SO42- |
Cr(OH)3 |
(d18) |
Cr2(SO4)3 | Chromium(?) sulfate (3 sulfates have a total charge of 6-, so two chromiums must have 6+. So what is the charge of just one chromium?) |


When chemists see a colored powder, they often start thinking of the possible metal ions that may be responsible for that color. Color can also be the result of organic plant pigments or some other organic (carbon-based) dye. However, if these organic pigments or dyes are ruled out, then they focus on the possible metal ions that give the powder its color.
Pigments from plants or animals (like insects) and other organic pigments (like food dyes) are more susceptible to heat. Remember what happened to sugar in lab 4 when you heated it in a flame? It would melt, boil, then decompose into a black mass. That often happens with organic pigments. Inorganic pigments (like metal salts) hold up better to heat.

Glass is colored by adding metallic oxides while it is in a molten state. For example, copper oxides (CuO and Cu2O) produce green glass. Cobalt oxides makes blue glass. Gold or rubidium oxides produce red glass. Lead and chromium oxides can make yellow glass.
Stain glass makers know what metal ions will create certain colors.

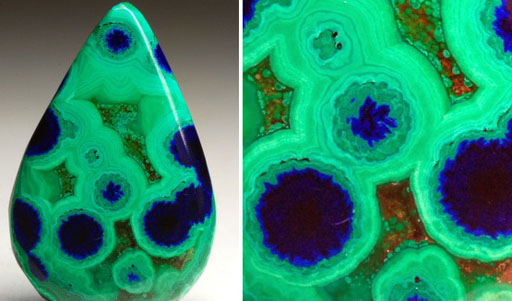
If you are looking for copper ore, you should look for green or blue colored rocks. In the photo is a polished piece of ore with two types of copper ores. One is called malachite and the other is azurite. Both have a mixture of copper(II) carbonate, CuCO3, and copper(II) hydroxide, Cu(OH)2.

House paints, car paints, and paints for artists also often contain metal salts.
Some oil paint names are cadmium yellow, cadmium red-orange, cobalt blue, and titanium white. Just from the names you can tell that metals are responsible for the colors. Also, because cadmium has more than one color, you realize that metals can have more than one color. Earlier in this lab, you saw that some metals can have more than one charge. For example, iron was shown as Fe2+ and Fe3+. The different charges (also called oxidation states) can affect the color.
One metal, chromium, creates many colorful compounds. The name "chromium" comes from "chromo" meaning "color".
Chromium is unusual because it can exist with 8 different charges (oxidation states). So that usually affects the color of the compound that it is in. The photo is of sodium chromate (Na2CrO4). The chromium atom has a +6 charge.
Color name |
Color |
Metal ions that are associated with these colors. The colors vary somewhat depending on what non-metals they are bonded with. The color also changes if another metal is present. The Roman numerals indicate the charges. |
| Brown | chromium(V), iron(II), copper(II)-anhydrous, cadmium(II), manganese (II & III) | |
| Red | chromium(I), chromium(II), chromium(III), cobalt(II), cadmium(II), lead(II & IV), vanadium(IV) | |
| Pink | manganese(II) | |
| Orange | cadmium(II), lead(IV) | |
| Yellow | chromium(VI), cadmium(II), lead(II), iron(III), vanadium(V) | |
| Green | nickel(II), copper(II), chromium(III) | |
| Dark green | vanadium(III), copper(II)-hydrated, chromium(III), manganese(II) | |
| Pale green | iron(II)-hydrated, nickel(II), copper(I) | |
| Blue-Green | copper(II), vanadium(IV) | |
| Blue | cobalt(II)-anhydrous, copper(II)-hydrated, vanadium(IV) | |
| Violet | chromium(III), cobalt(II)-hydrated, vanadium(II) | |
| Dark violet | manganese(VII), |
The below table lists the colors that are associated with a particular metal. Remember, these are not the color of the metal, but the compounds that have the metal in them. The negative ions that are bonded to the metal ions are often oxides (O2-), chlorides (Cl-), carbonates (CO22-), hydroxides (OH-), and sulfides (S2-).
| Metal ion | Brown | Red | Pink | Orange | Yellow | Green | Dark green | Pale green | Blue-green | Blue | Violet | Dark violet |
| Cadmium | ||||||||||||
| Chromium | ||||||||||||
| Cobalt | ||||||||||||
| Copper | ||||||||||||
| Iron | ||||||||||||
| Lead | ||||||||||||
| Manganese | ||||||||||||
| Nickel | ||||||||||||
| Vanadium |

A rainbow of colors. From left to right, aqueous solutions of: cobalt(II) nitrate, Co(NO3)2 (red);
potassium dichromate, K2Cr2O7(orange); potassium chromate, K2CrO4 (yellow);
nickel(II) chloride, NiCl2 (green); copper(II) sulfate, CuSO4 (blue);
potassium permanganate, KMnO4 (violet).

Your kit has copper(II) sulfate and iron(II) sulfate. Take a look at them.
e1) What color would you say copper(II) sulfate is?
e2) What color would you say iron(II) sulfate is?

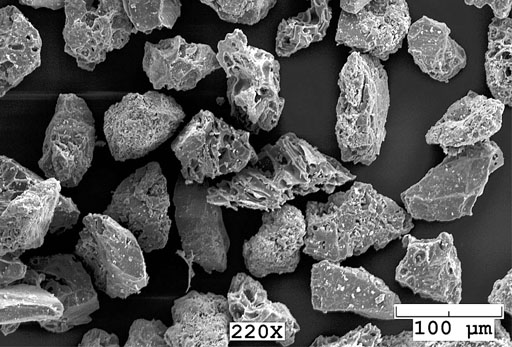
A scanning electron microscope can take black and white photos of very small objects. As the microscope creates these images, it can also give a readout of the elements that these particles are made from. Let's say these particles were found adhering to the murder weapon found at a crime scene. The particles are too small to be been with the human eye or a normal light microscope, so the color of these particles is not known. However, the scanning electron microscope reports that these particles contain the elements silicon, nickel, oxygen, and chlorine. Silicon and oxygen are the elements in silica (SiO2), which is in glass and sand. These are colorless. However, there are two other elements found.
e4) If more of this powder is on the suspect, what color would you tell the detectives to look for?
e5) If you exclude silicon and oxygen, what metal compound (metal salt) would you suspect is present in these particles?

A vanadium battery is great companion for wind generators. If there is excess electricity from excess wind energy, it can charge the vanadium battery by sending electrons on the right-side of the battery, which will convert V3+ into V2+. At the same time it takes out electrons from the left side which turns V4+ into V5+. From one of the charts above, you learned that vanadium had a lot of colors depending on its charge. The left side of this battery uses V4+ and V5+ ions. The colors the artist used for that side were yellow and blue. The right side of the battery involves V2+ and V3+ ions. The colors the artist used were green and violet.
e6) Did the colors that the artist used to make this diagram fit the actual colors that these ions have?

Some metals don't have a color when they are in a compound (in other words, the compound is white), but if subjected to a flame, they often will emit a color that helps to identify them. Flame tests can be done by placing a small amount of the metallic salt onto a small loop of platinum wire which is connected to a rod. The platinum loop is then held in the flame. The right side of this image is showing that technique for 3 metallic salts. The left salt is creating a yellow flame, the middle one is making a blue flame, and the right one a red flame. A platinum loop tool was considered for the kit, but it was too expensive. However, the other technique is to place the metallic salt powder on a watch glass along with some alcohol. As the alcohol burns, some of the salt gets hot enough to emit light. That technique is shown in the left side of this image.
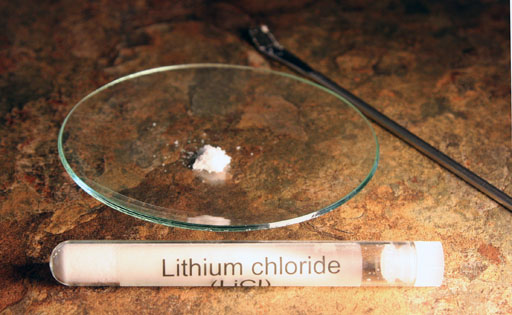
Use the microspatula to remove a small amount of lithium chloride from its test tube. Push the powder into a small pile in the center of the watch glass.
Notice this powder is white. So the metal lithium ion in lithium chloride is colorless. However, if subjected to heat, lithium does produce a color. Chlorine also produces a color, that that "color" is in the ultraviolet region of light. So it's invisible to our eyes. Lithium produces light in the visible light range.


Whenever there is a flame applied to some liquid or solid, it's important to put on the goggles. You never know if liquid or solid particles will fly into the eyes because of the heat. So put on your goggles now.
Using the lighter that is in your kit, you can use its flame to heat up the lithium chloride. The normal color of the butane lighter's flame is blue that turns to yellow. You will notice that by holding the flame onto the lithium chloride that a new color will be in the flame.
f1) What new color do you see?
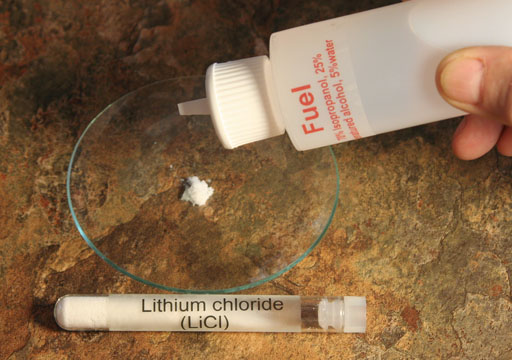


In this image, the alcohol has burned down so only alcohol coming up through the lithium chloride is burning. Notice the additional color in the flame.
f2) What name would you give this extra color that you see in the alcohol flame?
The lithium chloride powder appears to be boiling, but that's only the water (and perhaps alcohol) that is boiling. The Fuel alcohol has some water in it.
When done, let the watch glass cool and then rinse the watch glass off in the sink. Dry it so that it is ready for the next powder.
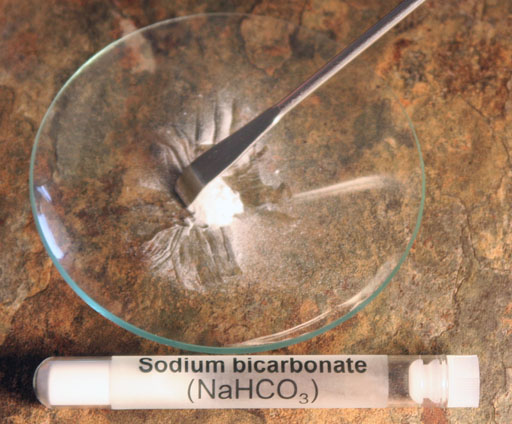
You are now going to try to another white powder in your kit. It is sodium bicarbonate (NaHCO3), also called sodium hydrogen carbonate.
Place some in the center of the watch glass and rake it into a pile.
Unlike lithium chloride, holding a lighter's flame next to sodium bicarbonate did not produce any extra colors. However, using the alcohol method does usually get results.
So add some of the alcohol burner fuel like you did with lithium chloride. If you don't have this fuel, then 91% rubbing alcohol should work.

As the alcohol burns and the flame gets narrower, the you should see some flashes of a color. This image doesn't show a flash of color. They were too fast to capture in a photograph.
I suspect some of the sodium bicarbonate is decomposing which produces some carbon dioxide and water vapor. Those gases may push grains of sodium bicarbonate into the flame which then causes the sodium to flash its characteristic color.
f3) What is the color of the flashes that you see?
Add more fuel to the sodium bicarbonate and light it again. This time take a photo of the flame over the sodium bicarbonate.



Locate the iron(II) sulfate that is in your kit. You are going to put just a couple of grains of it in the 25 mL graduated cylinder.
Also locate the test tube that has the strips for testing iron.

Also, find the color chart in your kit. It's usually behind the back panel.
The instructions for testing iron is to submerse the paper into the water and swirl it around 3 times. Then wait 10 seconds, then check the color on the iron test strip with the colors on the color chart.

Either use the microspatula or just tap on the test tube of iron(II) sulfate to get just a few grains into the 25 mL graduated cylinder. In the image we got two regular size grains and two tiny grains of iron(II) sulfate into the 25 mL graduated cylinder.

Add purified water to dissolve the few grains of iron(II) sulfate. Bring the water level up to 20mL.
Stir the water with the glass rod so the dissolved iron(II) sulfate is mixed thoroughly in the water.

Dip one of the iron test strips into the water in the 25 mL graduated cylinder. Remember to swirl it around 3 times. Then bring it to the color chart where the iron colors are shown.
Even though there were only a few small grains of iron(II) sulfate in the water, the test strip should detect it.
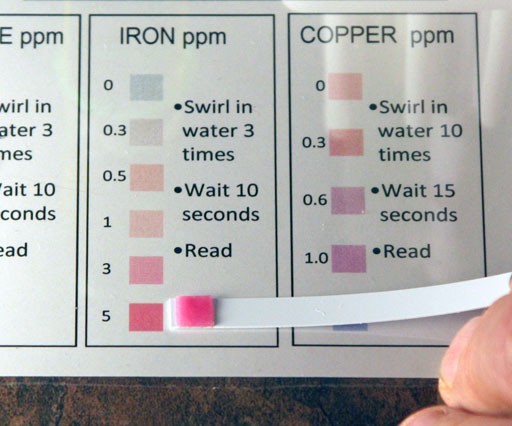
After dipping the iron test strip into the water, the test strip is compared to the colors on the chart. In this example, the few grains of iron(II) sulfate causes the test strip to read at the highest level, which is 5 parts of iron per 1 million parts of water (ppm). If we get a reading at the top of the scale, then it could actually be higher than 5 parts per million. To bring the reading onto the scale (less than the 5ppm), we need to dilute the water that has the iron(II) sulfate.
g1) What reading for iron does your test strip show?
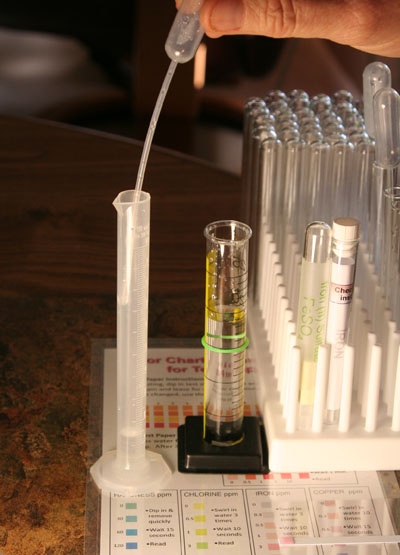

Add purified water to bring the water level up to 10 mL. At this point we have diluted our iron to have a concentration that is 10 times less than it is in the 25 mL graduated cylinder.
After you bring the water up to the 10 mL mark, use the disposal pipette that you used to transfer the 1 mL to mix the liquid in the 10 mL graduated cylinder. To do that, draw the water in the 10 mL graduated cylinder into the pipette and squeeze it back out. Do that about 5 times to mix the 1 mL that was added first with the 9 mL of purified water added afterwards.
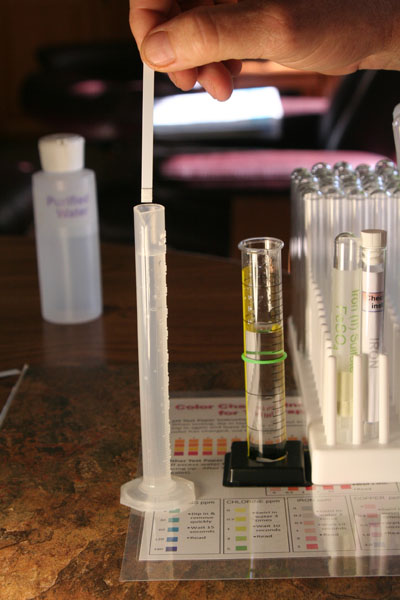
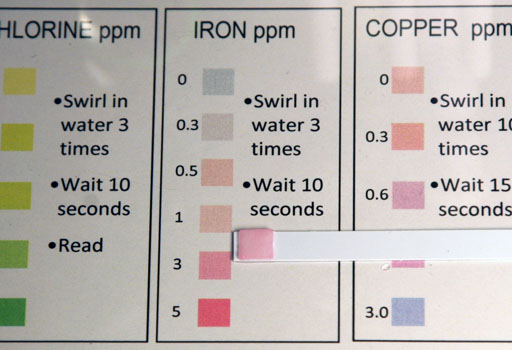
After being diluted to be 10 times less concentrated, we see that the color on the iron test strip is now below the red 5ppm. It looks close to 3ppm. That means the water in the 25 mL graduated cylinder, which has 10 times the concentration of iron, was actually 30ppm (3ppm x 10) and not 5ppm. The lesson is if a reading is at the top of the scale, you can't be sure that it is accurate because a higher concentration could give the same color. So by diluting it and bringing it below the top level, the concentration can then be accurately read.
If you had more grains of iron(II) sulfate than we did, then even after dilution, your color may still be at the red 5ppm.
g2) What is your reading for iron after this 10 fold dilution?
Take a photo of your test strip for iron.



Fill the smaller 50mL beaker with 10 mL of tap water. Locate the silver nitrate bottle. Remember silver nitrate stains. use the protective mat that comes with the kit. You may also want to wear gloves.
Add two drops of silver nitrate to the 10 mL of water in the beaker. This will dilute the nitrate that is in the silver nitrate.

Because we are using tap water, you are apt to see some white precipitate form when you add the drops of silver nitrate. This is a chemical change as covered in lab 4. If you remember, silver nitrate will react with the sodium chloride in tap water to make silver chloride (AgCl). Silver chloride is the white precipitate. Below is the reaction again showing the dissolved sodium chloride ions reacting with the dissolved silver nitrate ions.
Na+(aq) + Cl-(aq) + Ag+(aq)+ NO3-(aq)→ AgCl(s) + Na+(aq)+ NO3-(aq)
Since nitrate is what we are interested in testing, we are happy to see that the nitrate is not affected by this reaction. If we were trying to measure silver ions, then using tap water to dilute the silver nitrate would have been a problem because the chloride and silver ions would form the solid silver chloride precipitate, which would pull the silver ions out of the solution.
Swirl the water in the 10mL beaker so as to mix it up thoroughly.

Take the nitrate test strip and immerse it in the tap water that had the 2 drops of silver nitrate added. You have to bend the test strip so both pads are immersed. These strips test for nitrate (NO3-) and nitrite (NO2-).
Leave it immersed for 2 seconds then take out and place on the color chart next to the colors for nitrate and nitrite.

Wait a minute before you compare the colors. This test strip is showing that nitrate is about 5 ppm (parts per million). The nitrite (bottom pad) is zero. Nitrate levels of 10 ppm or less is considered satisfactory in drinking water.
g3) What is your reading for nitrate (top row)?
(Note: The ppm values got shifted to the left in this color chart. So be careful when reading them).
Take a photo of your test strip for nitrate.

Locate the test tube with the copper test strips.
In lab 2, you learned about the TDS meter which measures the total dissolve solids (mostly salts) that was in water. The measurement was also in parts per million. Remember, in cases of a solid dissolved in water, ppm is the same as milligrams per liter.
In the last experiment of this lab, you will create a solution of copper(II) sulfate that is 2 ppm copper. In other words, a concentration of 2mg of copper ions per 1 liter of water. You will also test this solution with the copper test strip in your kit.

Find the test tube with copper(II) sulfate. Remember, copper compounds are often blue, so they are easy to spot.
Weigh out a little more than 0.10 grams. In the picture, we weighed 0.14 grams. It was 3 crystals of the copper(II) sulfate. Actually, this is the hydrated form of this copper salt which has 5 water molecules attached. So the full name is copper(II) sulfate pentahydrate.
g4) What is the mass of copper(II) sulfate pentahydrate that you weighed out?
The reason we want more than 0.10 grams is to get two significant figures of accuracy. If we only measured out, let's say, 0.07 grams, then that only has 1 significant figure. By having 0.10 grams or a more, we get 2 significant figures of accuracy which justifies a more accurate final concentration.
Of the 0.14 grams weighed out in our sample, only a part of that mass is copper. The full formula is CuSO4•5H2O. Copper is just one of the elements. Looking at the Periodic Table, we find that copper weighs 63.546 grams per mole of copper atoms. Sulfur weighs 32.065 grams. Oxygen weighs 15.999 grams, but the formula shows 9 atoms of oxygen. So that is 15.999 x 9 = 143.991 grams. There is also 10 hydrogen atoms, which weighs 1.008 grams. So that is 10 x 1.008=10.08 grams coming from hydrogen. All of these add up to 249.69 grams. So copper's contribution is only 63.546 grams. So the fraction of the grams that copper makes is 64.546g/249.69g = 25.45%. So 25.45% of 0.14 grams is the mass of just the copper. That equals to 0.03563 grams of copper (without rounding) and 0.36 grams (rounded). So even though we weighed out 0.14 grams of copper(II) sulfate pentahydrate, we actually only weighed out 0.036 grams of copper. Turning that to milligrams of copper we get 36 milligrams (multiply by 1000). To find the milligrams of copper you weighed out, multiply the mass of your copper sulfate by 0.2545, then multiply by 1000. Then round to 2 significant figures. Your value should be 25 mg if you weighed out 0.10 grams. It will be higher if your grams was higher. Every time you read 36 mg below, that is referring to the sample we did that weighed 0.14 grams. Your milligrams is likely different.
g5) What is the mass of copper in your sample as calculated ( g4 x 0.2545 x 1000)?
If we wanted to make a solution of 2 parts per million (or 2 mg per Liter) concentration of copper, we would have to dilute the 36 milligrams (36 mg) of copper in the sample shown to the volume calculated below.
1 Liter x 36 mg = 18 liters
2 mg
The concentration of 2 mg per liter was inverted so that liter was on top and milligrams on the bottom. That causes the mg in 36 mg to cancel and leaving liters as the answer. Logically, the calculation means that every time 2mg divides into 36 mg, there is another liter need.
Dissolving our 36 mg of copper (contained in the 0.14 g of copper salt) into 18 liters will produce a 2 ppm (2 mg/Liter) solution; however, we would have to come up with 18 liters (about 5 gallons) of distilled water. That's too expensive, plus we only need a small amount of the 2ppm copper solution. The approach to diluting without using too much water is to do multiple dilutions. We will start by dissolving the copper sulfate in a 10 mL graduated cylinder.
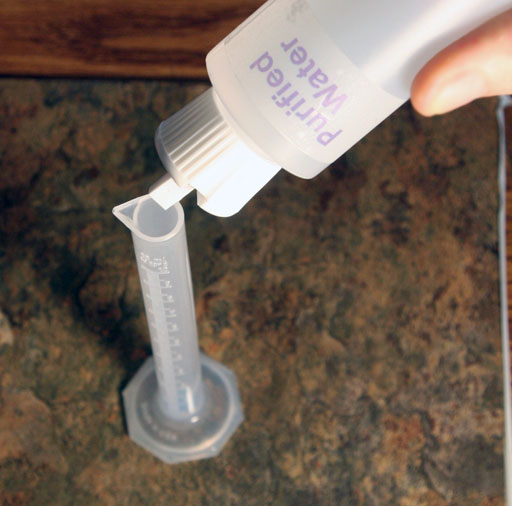
Transfer the copper sulfate to the 10 mL graduated cylinder. Then add purified water to fill the graduated cylinder to about half full. Use the glass rod to stir the solution until the blue crystals dissolve. That takes at least a minute or more.
After the crystals dissolve, add more purified water to bring the level up to the 10.0 mL mark.
At this point the concentration of the copper in the solution is much stronger than 2 ppm because we only dissolved it by 10 mL not 18,000 mL (18 Liters). The concentration now is 36 mg/10 mL, which is the same as 36mg/0.010 Liter
36mg = 3600 mg
0.010 Liter 1 Liter
Dissolving the 36 mg of copper in 10 mL (0.010 liter) gives us a concentration of 3600 mg/Liter (or 3600 ppm).

Pour the 10 mL of the copper solution into a clean 50 mL beaker. Once in the beaker, swirl the copper solution a few times to make sure it is mixed well.

Using a clean disposable pipette, transfer some of the copper solution back to the 10 mL graduated cylinder until you have 1.00 mL of solution in the graduated cylinder.

The 50 mL beaker now has 9 mL of the copper solution in it. Pour that solution into the 100 mL graduated cylinder.
After your have poured out the copper solution, wipe out the 50 mL beaker with a paper towel so it's fairly dry.
Now return attention back to the 10 mL graduated cylinder that has the 1 mL of copper solution in it.
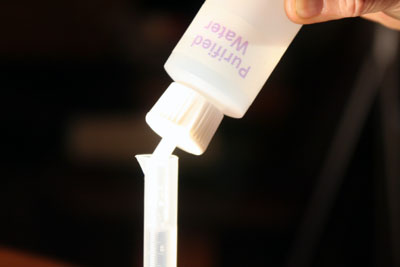
Fill the 10 mL graduated cylinder from the 1mL up to the 10.0 mL mark with purified water. Since you started with 1 mL of copper solution and then brought it up to 10 mL, you just now diluted it by a factor of 10. So instead of the 3600 ppm (3600 mg/L) concentration, you are now at 360 ppm (360 mg/L).

Like before, pour the 10 mL of the copper solution into the empty 50 mL beaker. This time you are pouring the diluted (360 ppm) copper solution into the 50 mL beaker.


Like the first time, the 9 mL of solution remaining in the 50 mL beaker is poured into the 100 mL graduated cylinder. So this gets added to the solution already in the 100 mL graduated cylinder. So now the 100 mL graduated cylinder has 18 mL of the excess copper solution.
Again, wipe out the 50 mL beaker with a paper towel so that it's fairly dry.
Now turn attention again to the 10 mL graduated cylinder.

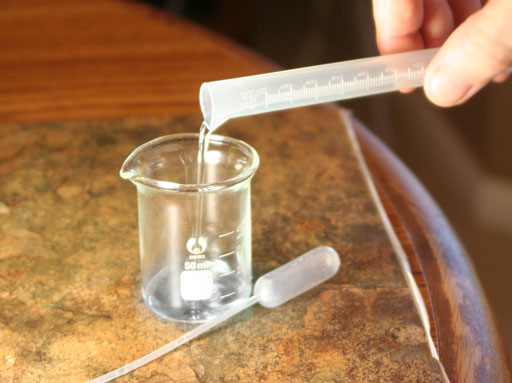
For the third time, pour out the diluted copper solution into the 50 mL beaker. This time the solution is 36 ppm copper. Swirl the solution to be sure it is mixed well.


Like the first time and second time, the 9 mL of solution remaining in the 50 mL beaker is poured into the 100 mL graduated cylinder. So this gets added to the solution already in the 100 mL graduated cylinder. So now the 100 mL graduated cylinder has 27 mL of the excess copper solution. (3 times 9 mL)
Again, wipe out the 50 mL beaker with a paper towel so that it's fairly dry.
Now turn attention again to the 10 mL graduated cylinder.

Also, like the first and second time, use purified water to bring the 1 mL copper solution up from 1 mL to 10 mL. This again, dilutes the copper solution by 10 fold. So the solution now went from 36 ppm to 3.6 ppm (3.6 mg/Liter). That's very close to the target of 2 ppm (2mg/Liter). To get from 3.6 ppm to 2 ppm, our volume needs to go up by the fraction of 3.6/2 which equals 1.8. In other words, if we increased the volume of 10 mL to 18 mL (10ml x 1.8), the concentration will drop from 3.6 ppm to 2.0 ppm. To increase to 18 mL, we just need to add 8 mL to our 10 mL.
It's not likely you started with 0.14 grams of the copper(II) sulfate. So for you to get to 2.0 ppm, the dilution will be different. Take your answer to (g5) given earlier, which is the milligrams of copper you started with. Divide that by 10 to get current copper ppm. For example, the answer should be 2.5 ppm if you weighed out 0.10 grams of the copper sulfate. It will be higher if you weighed out more.
g6) What is your current copper parts per million?
To find the final volume in milliliters that you need to dilute your copper solution to become 2 ppm, divide your answer to (g6) above by 2 and then multiply by 10. Your answer should be 12.5 mL or higher.
g7) What final volume (in mL) do you need for your copper solution to be 2 parts per million?
To get to the final volume pour the 10 mL back again into the 50 mL beaker. Then you will need to add enough water to bring that 10 mL up to the final volume.

After pouring the 10 mL of our 3.6 ppm copper solution into the 50mL beaker, we measured out 8 mL of purified water in the 10 mL graduated cylinder. Then we poured that 8.0 mL of water into the 50 mL beaker that already has 10 mL of the 3.6 ppm copper solution. The volume will now be 18 mL, so the concentration drops from 3.6ppm to 2.0 ppm.
Again, you will likely not be adding 8 mL but something else to get to your final volume you mentioned in (g7).
Now it's time to use the copper test strip to see if it comes close to measuring 2.0 ppm.


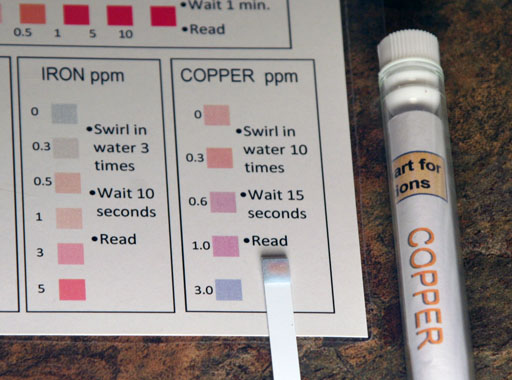
Wait 10 seconds and read the color. Here it looks like we are in between the 1.0 ppm and 3.0 ppm. So it looks like we got close to the 2.0 ppm (2 mg/Liter) that we targeted. Also, instead of using 18 liters (18,000 mL or about 5 gallons), we only used less than 50 mL of purified water. So that's pretty good.
g8) What parts per million does your copper test strip read?
Take a photo of your test strip for copper.


If you wish, you can copy the below summary into your email (or Word document) and type your answers after the descriptions. The required photos can either be attached to the email or inserted in the Word document if going that route. Try to keep each image under 2 megabytes. If you are in section 20598, which has a start date of Aug. 19th, email your lab report to Quinn Thacker at QRT2004@yahoo.com. If you are in section 20930 which has a start date of Sept. 9th, then email your lab report to Loree Cantrell-Briggs at lor2060912@phoenixcollege.edu. Be sure to title the email "Lab 6".
Lab 6: Lab Report
a. Binary Compounds (with covalent bonding)
Write the name if formula is given. Write formula if name is given:
a1) SiCl4 :
a2) SiO2 :
a3) SiS2 :
a4) Phosphorus trifluoride :
a5) PCl3 :
a6) triphosphorus pentoxide :
a7) P2S3 :
b. Binary Compounds (with ionic bonding)
Write the formula for the binary compound formed by the two ions shown. Then write its name. (Note, these chemical equations are not balanced.)
b1) Na+ + S2- → ? :
b2) Name of (b1):
b3) Mg2++ S2- → ? :
b4) Name of (b3):
b5) Mg2++ P3- → ? :
b6) Name of (b5):
b7) Al3++ Cl- → ? :
b8) Name of (b7):
b9) Fe2++ N3- → ? :
b10) Name of (b9):
b11) Fe2+ + S2- → ? :
b12) Name of (b11):
b13) Ag+ + S2- → ? :
b14) Name of (b13):
b15) Ag+ + P3- → ? :
b16) Name of (b15):
Write the formulas for the following chemical names.
b17) Potassium sulfide = ? :
b18) Silver bromide = ? :
b19) Calcium chloride = ? :
b20) Nickel(II) sulfide = ? :
b21) Magnesium phosphide = ? :
b22) Calcium oxide = ? :
b23) Iron(III) iodide = ? :
Write the chemical name for the following formulas
b24) Cu3P = ? :
b25) NaH = ? :
c. Ternary Compounds (made from 3 elements)
Write the formula for the compound formed by the two ions shown. Then write its name.
c1) NH4+ + SO42- → ? :
c2) Name of (c1):
c3) Mg2+ + CO32- → ? :
c4) Name of (c3):
c5) Mg2+ + SO42- → ? :
c6) Name of (c5):
c7) Mg2+ + PO43- → ? :
c8) Name of (c7):
c9) Al3+ + NO3- → ? :
c10) Name of (c9):
c11) Al3+ + PO43- → ? :
c12) Name of (c11):
c13) Cu2++ SO42- → ? :
c14) Name of (c13):
c15) Fe3+ + NO3- → ? :
c16) Name of (c15):
c17) Fe3+ + PO43- → ? :
c18) Name of (c17):
c19) H+ + NO3- → ? :
c20) Name of (c19):
c21) H+ + SO42- → ? :
c22) Name of H3PO4:
Write formulas for the following ternary compounds.
c23) Potassium sulfate:
c24)
Ammonium bromite:
c25) Zinc nitrate:
c26)
Nickel(II) sulfite:
c27)
Iron(II) carbonate:
c28) Magnesium hydrogen phosphate:
c29) Calcium chlorate:
c30) Cobalt(III) acetate:
d. Names and formulas of ionic compounds made from parent acids and bases. (Note: The following chemical equations are not balanced.)
d1) Name for NaCl :
d2) HI(aq) + KOH → H2O + ? :
d3) Name for (d2):
d4) H2CrO4(aq) + ? → K2CrO4 + H2O:
d5) Name for CaCO3 :
d6) Name for H2SO4(aq):
d7) H2SO4(aq)+ ? → H2O + MgSO4 :
d8) Name for MgSO4 :
d9) Formula for Cobalt(II) chloride :
d10) Formula for Sulfuric acid :
d11) Anion in sulfuric acid :
d12) Cation in Fe(OH)2 :
d13) Name for FeSO4 :
d14) Formula for hydrochloric acid :
d15) Anion in hydrochloric acid :
d16) HCl(aq)+ ? → H2O + NiCl2 :
d17) Name for NiCl2 :
d18) Cation in Cr(OH)3 :
e. Using the color of a salt to help identify certain metal ions.
e1) What color would you say copper(II) sulfate is?
e2) What color would you say iron(II) sulfate is?
e3) Face painting using metallic salts is not a good idea because many of these metallic salts are toxic. However, if a metallic salt was used to paint this boys face, which metals from the above chart would be suspected as being present?
e4) If more of this powder is on the suspect, what color would you tell the detectives to look for?
e5) If you exclude silicon and oxygen, what metal compound (metal salt) would you suspect is present in these particles?
e6) Did the colors that the artist used to make this diagram fit the actual colors that these ions have?
f. Flame Tests: Using the color of a salt in a flame to identify certain metal ions.
f1) What new color do you see?
f2) What name would you give this extra color that you see in the alcohol flame?
f3) What is the color of the flashes that you see?
Send photo of the sodium bicarbonate in alcohol flame.
g. Use chemical test strips to help identify various ions and determine their concentration
g1) What reading for iron does your test strip show?
g2) What is your reading for iron after this 10 fold dilution?
Send photo of your test strip for iron.
g3) What is your reading for nitrate (top row)?
Send photo of your test strip for nitrate.
g4) What is the mass of copper(II) sulfate pentahydrate that you weighed out?
g5) What is the mass of copper in your sample as calculated ( g4 x 0.2545 x 1000)?
g6) What is your current copper parts per million?
g7) What final volume (in mL) do you need for your copper solution to be 2 parts per million?
g8) What parts per million does your copper test strip read?
Send photo of your test strip for copper.
Send a photo of yourself holding up the test tubes that hold the copper, nitrate, and iron test strips.
If you wish, you can copy the below summary into your email (or Word document) and type your answers after the descriptions. The required photos can either be attached to the email or inserted in the Word document if going that route. Try to keep each image under 2 megabytes. If the first letter of your last name is between A and G, send your lab reports to Loree Cantrell-Briggs at Loree.Cantrell-Briggs@phoenixcollege.edu If the first letter of your last name is between H and Z, send your lab reports to Quinn Thacker at QRT2004@yahoo.com. Be sure to title the email "Lab 6".