
One measure of the quality of the water in lakes, rivers, and streams is the total amount of solids dissolved in the water. High amounts of dissolved solids can indicate poor water quality. The same is true for drinking water.

Objective 1: Learn the meaning, applications, and measurement techniques for "total dissolved solids".
Basic Meaning: "Total Dissolved Solids (TDS)" is the concentration of the dissolved chemicals in a sample of water. Before dissolving, these chemicals could have been a solid or a liquid.


Objective 2: Determine the TDS (Total Dissolved Solids) value of various samples using both of the below methods.
2a. Find TDS using a gravimetric method.
Gravimetric means "by weighing". Balances require gravity to weigh something. You will weigh the total dissolved solids after water is boiled away. This will be done using just one water sample.
2b. Find TDS using electrical conductivity.
This is rather easy to do. Just dip the TDS probe into the water and the TDS meter will measure how well the water conducts electricity. It then converts that to concentration of total dissolved solids.

River, lake, and stream testing.
Pure water has nothing dissolved in it. So pure water has zero total dissolved solids. However, when minerals, salts, and pollutants dissolve in water, then the total amount of these dissolved solids gives an indication of the water's quality. The Environmental Protection Agency, for example, would measure total dissolved solids (TDS) in lakes, rivers, and streams to monitor water quality.

Swimming pool and spa maintenance.
High TDS indicates hard water, meaning there are a lot of dissolved minerals that will form scale (white crusty mineral deposits made mostly of calcium carbonate) on the sides of the swimming pool or spa and the insides of pipes. Monitoring TDS can allow intervention before scale forms.

Agriculture and hydroponics (hydroponics is the science of growing plants without soil).
Moisture in soil that has high salt levels will not move into the plants' roots, causing drought symptoms even when there is plenty of water present. A TDS meter can see if the water for the plants is too salty.
Using a meter that measures TDS is also useful in keeping track of the level of nutrients in the water. Most of the nutrients for plants increase the TDS levels (more nutrients are dissolved). So in these cases, a high TDS level indicates plenty of nutrients are present. This meter can also check the quality of water that is being brought in to water the plants. In looking at water coming in, high TDS might indicate too many minerals (hard water). So the grower may want to lower the TDS by removing the excess minerals before adding nutrients.
The TDS meter is on the left. An electrical conductivity (EC) meter is on the right. The EC meter is basically measuring the same thing but reports values using different units of measurement.

Aquarium maintenance
TDS (total dissolved solids) is a measurement that can help track the levels of dissolved waste and dissolved minerals. When TDS levels are too high, the aquarium water is pumped through various filters to remove the dissolved waste and dissolved minerals. These filters are often reverse osmosis membranes that allow water to pass but little of anything else.

Aquaculture
Aquaculture is the farming of fish, oysters, or seaweed in controlled environments.
TDS levels are monitored because high levels of TDS can kill young fish.
![reverse osmosis plant]](reverseOsmosis2.jpg)
Water treatment plants and home water use
Water that has high TDS values will taste salty, metallic, or bitter. The Environmental Protection Agency (EPA) sets the maximum level of total dissolved solids for drinking water to be 500 milligrams (half a gram) of dissolved solids for every liter of water.
These dissolved solids are removed using reverse osmosis membranes. As mentioned earlier these membranes allow water to pass through but block large atoms, larger compounds, and microscopic particles that make up dissolved solids. These membranes also block toxic metals and other toxic substances.
Reverse osmosis systems are used in the home, water plants, and at places that dispense drinking water (usually at 25 cents a gallon)
Source and make up of dissolved solids

Rain water has no dissolved solids. So it has zero TDS. However, when it contacts the ground, the rain will dissolve fertilizers, salts and minerals, animal waste, pesticides, plus other chemicals that may be on the ground from cars and industrial pollution. Even decaying plants have chemicals that get dissolved. Water can also pickup more solids as it passes through copper pipes. So these dissolved solids can be a multitude of chemicals.
The most common chemicals counted in TDS tests are salts like sodium chloride (table salt), calcium chloride (salt placed on icy roads) and fertilizers like ammonium nitrate, various phosphates, and various potassium salts (potassium carbonate, potassium chloride, potassium sulfate). There are also dissolved minerals like calcium carbonate (limestone) or magnesium carbonate and calcium sulfate (gypsum/drywall material) or magnesium sulfate (Epsom salts).
There are thousands of other chemicals in our water, but TDS looks at all of them as one group (one reading).
How is TDS measured?

Gravimetric method: To measure TDS using this method, the water sample is first passed through a filter that blocks anything bigger than 2 microns ( 2 micrometers or 2 millionths of a meter). This ensures the test measures dissolved solids not solids suspended in the water. Such things as sediment or specks of plant material are filtered out and therefore not counted in the "total dissolved solids".

Gravimetric method continued: A certain amount of the filtered water is then weighed out and the water is boiled away leaving the dissolved solids behind as a solid residue. This residue is weighed. This is called the gravimetric method because a balance is used. Balances need gravity to find the mass. So that's why it's called a gravimetric method.
In this lab you will be doing something similar, but instead of a Bunsen burner you will use an alcohol burner. Instead of a glass beaker, you will use an metal can. Your kit has a glass beaker but the metal can is unbreakable and disposable if it gets damaged. Roll cursor over image to see the setup done in this lab.
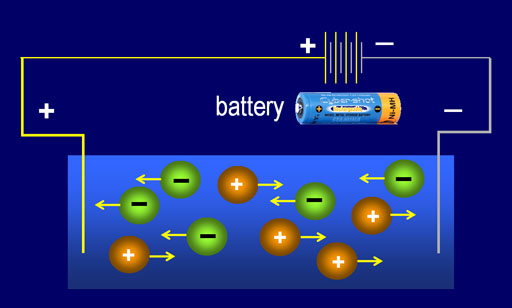
Electrical Conductance Method: The other way TDS is tested is by measuring how well the water sample conducts electricity. If the dissolved solids are salts and minerals, they would have dissolved (disassociated) into plus and minus ions. This allows electricity to pass through the water. For example, sodium chloride (NaCl) becomes Na+ and Cl- when dissolved in water. The negative chloride ions (Cl-) will be attracted to the + side of the battery terminal to give up their electrons, and the positive sodium ions (Na+) will be attracted to the negative terminal to pick up electrons. In this manner, the ions will allow electrical current to pass through the water. The more electrical current flows through the solution if there are more ions in the solution. More ions mean there are more dissolved solids, so TDS goes up.
Electrical conductance is the measurement of how much a material (liquid, solid, or gas) conducts electricity.
Electrical Conductance Method: Measuring electrical conductance with a meter is a quick way to get a measurement of the total dissolved solids. There are two meters of this type. There is an electrical conductance meter (EC meter) and a TDS meter.
The electrical conductance method does miss chemicals like sugar, alcohol, glycerin, and antifreeze. These chemicals do not form ions and therefore do not help conduct electricity, so they aren't measured using one of either of these meters; however, chemicals like these can be measured using the gravimetric method by weighing the water sample before, during, and after the water is boiled away.

The EC meter (electrical conductivity meter) measures in siemens (named after German inventor Ernst Siemens). On this meter the readout is 1220 and notice the unit is µS, which means microsiemens (one millionth of a siemens).
Siemens is directly related to the total dissolved solids. Using a calibration solution, these meters can be adjusted to read the proper electrical conductance (in siemens) or the proper TDS (total dissolved solids measured in parts per million or milligrams per liter).
A TDS meter is actually an electrical conductivity meter (EC meter) that calculates TDS from the siemens value and reports the TDS concentration in mg/L. That's why you see meters that are dual TDS and EC meters.

Here are the concentrations written as fractions. The fraction bar is the "per".
parts = grams ÷ 1000 = milligrams = milligrams
million 1,000,000 grams ÷ 1000 1,000 grams Liter
The math shows us going from the generic "parts per million" to a more specific "grams per million grams." This is reduced by dividing numerator and denominator by 1000 to get milligrams per 1,000 grams. Knowing that a liter of water weighs 1000 grams, "Liter" replaces the 1,000 grams. So if you are given the concentration of total dissolved solids as 435 ppm, you can change that to 435 milligrams per Liter.
TDS meters are calibrated at a particular temperature. Usually 25°C (77°F). If the solution is hotter than 25°C, then the reading will be higher than it should be. Notice that this meter has a temperature compensation adjustment. Your meter does not. So you will be doing some calculations that adjusts the TDS reading to compensate for temperatures higher or lower than 25°C. The meter in your kit was tested and found that you need to subtract 1.90% (0.0190) of your TDS reading for every degree over 25°C and subtract 1.90% of your TDS reading for every degree below 25°C. For example, if your tap water was 30°C and your TDS reading was 480 mg/L, then you would subtract 25°C from 30°C to get 5°C. Multiply 5°C x 1.90% per °C to get 9.50%. Multiply 9.50% (0.0950) times 480mg/L to get 45.6 mg/L. Now subtract the 45.6 mg/L from 480 mg/L. That gives you 434 mg/L, which is the TDS value corrected for a difference in temperature (different from 25°C). Here is a formula of these steps:
Reading - [0.0190 x Reading x (Temp-25)]
If your solution was colder than 25°C, also find the difference from 25°C. For example, if the solution was 21°C, then 25°C-21°C= 4°C. Multiply 4°C x 1.90% per °C to get 7.60%. Take 7.60% of 480 mg/L to get 36.48mg/L. Since 21°C is colder 25°C, you don't subtract, you add. Add 36.48 mg/L to the 480 mg/L to get 516.48 mg/L. Round that to 516 mg/L (3 significant figures). Again, if the temperature is over 25°C, you need to subtract 1.90% for each degree over. If below 25°C, you add 1.90% for each degree under. Here is a formula of these steps:
Reading + [0.0190 x Reading x (25-Temp)].
1. Once a solid is dissolved in water, is it still solid?
2. If sugar is dissolved in water, does it get measured using a TDS meter?
3. If sugar is dissolved in water, does it get measured using the gravimetric method for finding total dissolved solids?
4. If snow was melted and tested with a TDS meter, what level would you think it has?
5. If you take tap water and make ice cubes with it, does the TDS level change and why?
6. Would a bottling company like Coca-cola do TDS testing? (Why or why not?)
7. If a full glass of water sat outside in the sun for few days and half of the water evaporated, does the water in the glass now have higher, lower, or the same TDS level as the water when the glass was full?
8. Sodium nitrate is a common fertilizer. In water, this solid disassociates into Na+ and NO3-. Will these ions reduce or raise electrical conductance in water?
9. Which would have a higher TDS reading, water from a freshwater lake or water from the ocean?
(answers are at the very end of this lab).

TDS meters are popular because they are easy to use. There's just a few things to keep in mind in order to get accurate readings.
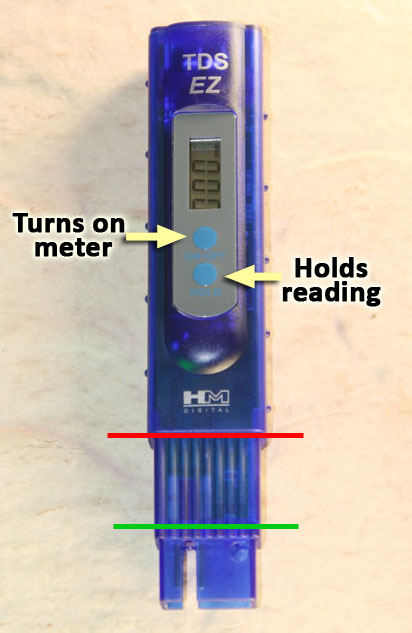
This is the TDS meter which is in your kit. The readout screen shows TDS in parts per million. Note, that the same numbers can also be read as milligrams per liter.
The top blue button turns on the meter. Use the same button to turn it off. If left on too long, the meter will turn itself off.
The bottom blue button freezes the readout so that you can remove the meter from the water and the readout doesn't change.
The meter needs to be submerged between the green line and the red line. Do NOT submerge the meter any lower in the water than where the red line is. The TDS meter is not water-proof.
The solution needs to cover the metal posts that measure the conductivity. If the meter is dipped to the green line, then the metal posts are covered.


To take a reading, simply dip the probes into the water being measured. Stir meter gently so that any bubbles clinging to the metal posts are dislodged. If you can see the screen, then you can get the reading for Total Dissolved Solids. If it's hard to see the screen, press the lower blue button once to freeze the reading on the screen, then bring the meter up close to you to read the screen.


The first thing to test is your tap water.
Fill the larger beaker (250mL beaker) between 50 and 100 mL with tap water from the cold water side.
Place the TDS meter into the beaker, and place the thermometer from the kit along side the TDS meter. Turn on the TDS meter (top blue button) and record the TDS reading. Now record the temperature. Remember to estimate to the nearest tenth of a degree. You can use the Fresnel lens magnifier to help read the temperature scale.
The TDS meter is calibrated to be accurate at 25.0°C. If your tap water is different from 25.0°C, then there needs to be an adjustment for the different temperature. Look at the math above in the "Math for TDS" section that shows how to do that. Again, if the temperature is over 25.0 °C, you need to subtract 1.90% (0.0190) of the reading for each degree over. If below 25.0°C, you add 1.90% of the reading for each degree under. In this example, the reading was 492 mg/L. The temperature was 28.9°C. That is over 25.0°C, so we need to compensate for the temperature difference by subtracting. The temperature difference is 28.9°C-25.0°C, which is 3.9°C.
compensation x temp difference x reading = correction amount to be added or subtracted
0.0190 x 3.9°C x 492 mg/L= 36.46
1 °C
492mg/L - 36.46 mg/L = 455.54mg/L rounded to 456 mg/L is the corrected TDS value.
So report both the TDS value that you read off of your meter plus the TDS value corrected for the temperature of your tap water.
Compare the corrected reading you calculated with the readings in the below chart. What category does your tap water fall into?
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|

Now check the TDS level of a beverage like a soda, juice, tea, coffee, or beer. Warning: If you choose a carbonated beverage, you will probably notice the readings jumping up and down. What do you think would cause that?


Rinse out the 250 mL beaker and fill it between 50 mL and 100 mL with a beverage of your choice. Put the thermometer in the beaker too. Record the temperature and the TDS value, which is in mg/L (ppm). To get the reading corrected for temperature, do the same calculations mentioned above and also report the TDS value corrected for temperature.
If your beverage was cold, you will notice the TDS values going up as the liquid temperature goes up. That doesn't mean the TDS values are changing because the dissolved solids in the beverage are not changing. It just means the warmer TDS values will have less adjustment for temperature.
To be accurate, record the temperature and the TDS values close to the same time.

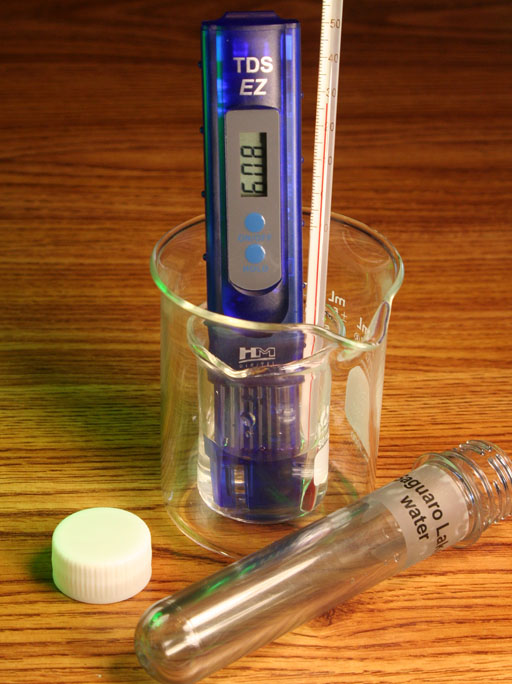
The kit also has a sample from Saguaro Lake which we want to test the TDS value. Unfortunately, there is only about 25 mL in your sample. If you pour it in the 250 mL beaker, the water level will not be deep enough to cover the probes in the TDS meter. What you can do is pour the 25 mL into the small 50mL beaker. The problem is that the TDS meter will tip over if in that small beaker. However, if you put the small beaker into the 250 mL beaker that will keep the TDS meter, small beaker, and thermometer from tipping over.
Record the temperature of the water.
Use the TDS meter to read the mg/L of dissolve solids in that water. Record that value. Then do the calculations that corrects for any temperature away from 25°C. Report the value corrected for temperature.
How does the TDS value for Saguaro Lake water compare to your tap water value?


Your kit has a solution of which contains a mixture of salt and sugar (and water of course).
The basic approach here is simple. You will need to measure out a known amount of the solution. Then boil the solution leaving a solid residue. The water actually doesn't have to boil, it just needs to evaporate.The boiling is just to speed up the evaporation of the water so you can see and weigh the solid residue sooner.
The residue left after water is boiled away is the dissolved solids. Weighing those dissolved solids and dividing that by the weight or volume of the solution, lets you find the Total Dissolved Solids concentration.

You will use the alcohol burner in the kit to boil away the water, but first read the instructions for using the alcohol burner. Those are in the back of the kit. You can also read it online in PDF format by following this link:
Use your back arrow in the browser to come back to this page.

Locate the metal can in your kit and weigh it to the nearest one hundredth of a gram. Record that mass.
The metal can should be sitting under the alcohol burner.

Locate the bottle labeled "Salt & Sugar solution". (It should be in the upper left side of the case.)
Earlier I said that any solution to be tested for TDS using gravimetric methods needs to be filtered through a 2 micron (2 micrometers or 2 millionths of a meter) filter. That has already been done.
You are going to measure the solution in two ways. One by measuring the volume and the other by measuring the mass of the solution.
To measure volume, pour the entire contents of the Salt & Sugar solution into the 100 mL graduated cylinder. Record the volume to the nearest tenth of a milliliter. Use the Fresnel lens magnifier if you wish. Remember to look straight across and read at the bottom of the meniscus.

Take the can off of the balance and pour the salt & sugar solution that is in the graduated cylinder into the metal can.
Weigh the can again with the salt/sugar solution in it. Record the mass of the can plus the salt/sugar solution. Subtract the can's mass (done earlier) from this mass to find the mass of just the salt/sugar solution. At this point you have the salt & sugar solution measured in both volume (mL) and in mass (grams). These values will be close to each other because water weighs 1 gram per milliliter. However, the salt & sugar changes the mass somewhat.
The next task is the boil away the water. So that requires setting up the alcohol burner.

Remove the small metal cap that covers the copper wick on the alcohol burner. There will be a small piece of foam rubber under the cap that keeps it from falling off. Just set that aside to put back when finished with the burner.

Remove the lid of the alcohol burner by turning counterclockwise (just like normal lids). Fill the alcohol container to about 3/4 full with the denatured ethanol in your kit. (If your kit was mailed, you won't have the denatured ethanol in the kit. You can pick some up at a hardware store. Hopefully you can find a quart rather than buying a whole gallon.)

Place the lid back on by turning it clockwise. Tilt it side to side to make sure no alcohol is leaking out.

Remove the rubber stopper that blocks the holes in the copper coil.
There are glass fibers in the copper tubes that draw the alcohol up into the copper loop and out a few small holes. The rubber stopper blocks those holes and keeps the alcohol from leaking out (as least in theory).

This is the setup. Notice these items set on a one foot square piece of tile. The alcohol burner and stand gets hot when the flame is on for awhile. The tile will protect the table or counter top. The tile is in the back of the kit behind the pocket panel.
You will need the butane lighter from the kit and the metal tongs. Warning: The alcohol burner, metal stand, and metal can will all get quite hot when the flame has been on for a few minutes. Use the metal tongs to move anything.
(Yellow background means this is the part of the lab where safety is utmost).

Before lighting, be sure the metal shield is around the copper wick. The metal shield acts both as a block to wind and also directs the heat upward.
During the lighting of the burner, wear your goggles.

Use the butane lighter to light the alcohol lamp. The squeeze trigger on the butane lighter requires quite a strong pull to get the trigger to pull back and light the butane flame. Hold it for about 2 seconds onto the copper coil, then release the butane trigger. Look for a flame. Remember, an alcohol flame can be hard to see. If no flame, squeeze the trigger again on the butane lighter and heat another couple of seconds. If it doesn't light right away, swirl the alcohol burner gently to get some liquid and vapors to go into the copper coil, then try lighting it again. Repeat until you see a flame. If no flame, did you remember to put denatured alcohol in the alcohol base?

Notice that the flame is hard to see. Sometimes you have to put something black behind the burner to see the flame. Or you can put you hadn a few inches above the stand and feel if heat is rising from the flame.
This is where you need to warn anyone around you that there is a flame here and the whole setup will get very hot.

It will take about 30 minutes for most of the water to boil away and leave the salt behind. Like any fire, be sure to keep an eye on it. If you are not standing close to the burner, you can take your goggles off. If you go over to look into the can to check on the boiling of the solution, then put on your goggles while doing that.

If all the water boils away and you continue to heat it, the sugar will caramelize and turn brown or black. You don't want that to happen because some of the sugar will turn to smoke and float away. The sugar can even catch fire. Losing mass will give you the wrong mass for the total dissolved solids. To prevent this, instead of letting all of the water boil away, blow out the flame when the water is almost boiled away. Then let everything cool for about 3 minutes.

At this point or while the water is boiling take a photo of your whole setup. If you know how, set the photo size to about 2 megabytes. Or reduce the size of the image later if you have the software to do that.

After the flame is blown out, take a closeup picture looking down into the can at the salt and sugar residue (the dissolved solids).
If the water still hasn't all evaporated, light up the alcohol burner again and let it heat up the can again for a minute or two. Watch it closely. If the residue starts to brown, blow out the flame. If the water seems to be all evaporated, blow out the flame. Let everything cool down.
After 3 minutes of cooling set the metal can onto the tile and let it cool a couple more minutes. Carefully feel the metal can and see if it is room temperature.

If cool, turn on the digital balance and make sure it reads 0.00. Place the metal can with the salt and sugar residue onto the digital balance. Record the mass of the can plus residue.
Remember, something that is extra warm will cause the balance to give an erroneous reading. So if you see the digital readout continually rising or falling, the can may be too warm. Also, the values can change if there's a breeze blowing on the balance.
Record the mass. Let the can set out for an hour to let any remaining water evaporate, or you can also place it in the sun or in a warm oven to speed up the remaining evaporation. Let can cool and weigh it again. If the mass is within 0.03 grams of the first weighing, then you are finished. If the difference is more than 0.03 grams, then water is probably still evaporating. So let it sit another hour preferably in a warm place, and then weigh it again. When the mass is less than 0.03 grams different from the previous mass, then you can assume all the water is gone, and you have an accurate mass of the can with the dissolved solids residue. You can also let the can sit overnight at room temperature to let more water evaporate.
You now have enough information to find the TDS value of this salt & sugar solution. You need to subtract the mass of the can from masses that include the can's mass.
Mass of the metal can with the salt & sugar solution
- Mass of metal can
Mass of salt & sugar solution
Mass of metal can with the salt & sugar residue
- Mass of metal can
Mass of salt & sugar residue
Total dissolved solids is a concentration measurement. All concentrations are given as the amount of the solute (dissolved material) per a set amount of solution. The "per" is indicated with a fraction bar "/". So solute/solvent (solute divided by solvent) is a measure of concentration.
To get the concentration in ppm (parts per million), we put the parts (mass of residue, which is the dissolve solids) over the mass of the solution
In the solution used for taking photos, the mass of the solution was 59.84 grams. The mass of the residue was 1.27 grams. So we make it a fraction:
1.27 grams dissolved solids
59.84 grams of solution
The above fraction is a concentration but not in the format of parts per million. Here we have only 59.84 grams not a million grams. The plan is to change 59.84 grams to one gram then multiply by a million. See below for the problem solving steps.
1.27 grams residue x 1,000,000 = 21220 g dissolved solids = 21200 ppm
59.84 grams of solution x million million grams solution
The math is to take the 1.27 divide by 59.84 and then multiply by 1,000,000. The answer isrounded to 3 significant figures because the mass of the residue was only accurate to 3 significant figures. Again, the ppm means the parts (grams of dissolved solids) per 1 million parts (1 million grams) of solution.
Data can be converted to mg/L if we assume that one 1000 grams of solution is one liter, which is true for water and for dilute solutions.
21220 g dissolve solids ÷ 1000 = 21.22 g = 21.22 g x milli = 21,220 mg dissolve solids
million grams solution ÷ 1000 1000 g 1 Liter 0.001 1 Liter of solution
Notice we divide by 1000 to turn million grams into 1000 grams. Then 1000 grams of solution is considered to have a volume of 1 liter. Then dividing by 0.001 we turn grams into milligrams. 21200 mg/L is a very high TDS level. This is what you find in salt water aquariums.
Remember, you also measured the volume in mL. In the solution used for photos, the volume was 59.9 mL. So we can use the 1.27 grams of the residue to get the below concentration:
1.27 grams residue x milli x milli = 21,202 mg = 21,200 mg/L (rounded)
59.9 mL solution 0.001 0.001 1 L
The first "milli/0.001" is to cancel out the "m" in mL. The second "milli/0.001" is to add milli to grams to make milligrams. The final answer is rounded to 3 significant figures. Notice that we get the same answer for both methods when rounded to the proper number of significant figures.
Report the TDS of your salt & sugar solution both in ppm and in milligrams per liter.
If you wish, you can copy the below summary into your email (or Word document) and type your answers after the descriptions. The required photos can either be attached to the email or inserted in the Word document if going that route. Try to keep each image under 2 megabytes. If the first letter of your last name is between A and G, send your lab reports to Loree Cantrell-Briggs at Loree.Cantrell-Briggs@phoenixcollege.edu If the first letter of your last name is between H and Z, send your lab reports to Quinn Thacker at QRT2004@yahoo.com. Be sure to title the email "Lab 2".
A. Tap Water:
1. Temperature of your cold tap water:
2. TDS reading for cold tap water:
3. TDS reading of cold tap water corrected for temperature difference (calculated):
4. What category does your water fall into according to the chart?
B. Beverage:
1. What beverage did you check?
2. Temperature of the beverage:
3. TDS value of the beverage:
4. TDS value of beverage corrected for temperature difference (calculated):
5. Attach camera image of you holding the beverage or send it separately.
C. Saguaro Lake water:
1. Temperature of Saguaro Lake water:
2. TDS value of Saguaro Lake water:
3. TDS value corrected for Saguaro Lake water (calculated):
D. Gravimetric method for finding TDS:
1. Mass of empty metal can:
2. Volume of salt & sugar solution:
3. Mass of metal can with the salt & sugar solution:
4. Mass of salt & sugar solution (calculated):
5. First mass of metal can with the dry salt & sugar residue:
6. Final mass of metal can with the dry salt & sugar residue:
7. Mass of salt & sugar residue (calculated).
8. Attach or send camera image of the alcohol burner setup:
9. Attach or send camera image of your salt & sugar residue in the metal can:
10. Divide salt & sugar residue mass by solution mass and report TDS as ppm:
11. Divide salt & sugar residue mass by solution volume and report TDS as mg per liter:
Post-Lab questions and problems are on the Sapling Learning website. Those are graded automatically, so you will get your score on your Post-lab questions as soon as you finish them. http://www2.saplinglearning.com/
1. Once a solid is dissolved in water, is it still solid?
2. If sugar is dissolved in water, does it get measured using a TDS meter?