Lab #5: Water & Soil
Experiment #2: Creating and testing Filters

Understanding the strengths and weaknesses of filters
is important in knowing the quality of the water that comes out the filter.
Filters can do three things.
1) They can improve the quality of water.
2) They can do nothing.
3) They can make it worse.
It depends on what they are designed for and if
they are scientifically valid. In other words, many companies make their
products sound scientific but on closer scrutiny you are apt to find misinformation.




This graphic is from Lab 1. It says that activated charcoal is good for removing organic compounds. This includes pesticides, certain fertilizers, and many decay products from plants and animals. Organic compounds are usually responsible for bad tastes and smells. So activated charcoal can be good in helping there. What it is NOT good for are dissolved salts. These salts make water "hard." Dissolved salts are too small for the charcoal to trap, plus because salts are charged, water pulls on them and keeps them from sticking to the charcoal.
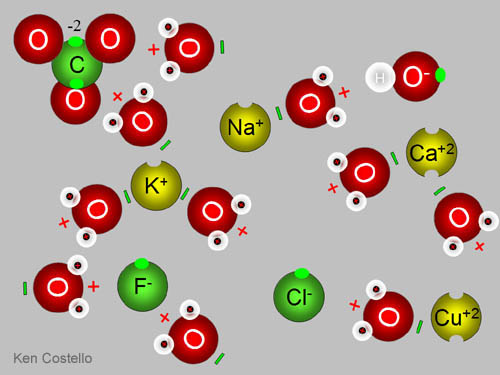
Dissolved salts have positively charged metal ions like sodium ion (Na+), potassium ion (K+), calcium ion (Ca+2), copper ion (Cu+/Cu+2), and dozens more metal ions. >>> The negative half of these salts are chloride (Cl-), fluoride (F-), carbonate (CO3)-2, nitrate (NO3)-2, hydroxide (OH)-, arsenate (AsO4)-3, and dozens more. >>>Notice in the picture, water surrounds the ions. The negative end of water will attracted the positive metal ions. The positive end of water the negative ions. The bites out of the positive metal ions means some other atom took an electron away. The bumps on the negative ions represent the extra electron(s) it has gotten from the metal ions.

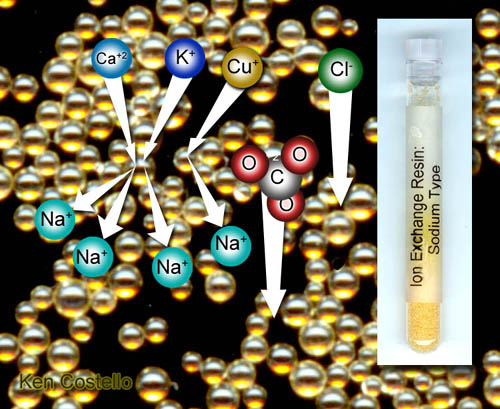
This type of resin is commonly used in home water softening
systems. Let's reveiws the pro's and con's.
Pro's: It removes the calcium and magnesium ions that interfere
with soaps and some cleaners.
Pro's: It removes some metals that are not healthy (copper, lead,
chromium, tin, and more).
Pro's: By passing concentrated salt solution (NaCl) through the
resin, the trapped metal ions can be flushed away as new sodium ions regenerate
the resin's ability to soften water. This is why we add bags of salt every
so often in our water softeners.
Pro's: Sodium salts are more soluble than most other salts. So
cleaning water spots and scale made from sodium salts is easier.
Con's: It removes all metal ions (minerals) except for sodium.
Some that are healthy like calcium. Potassium is considered healthier
than sodium. People who are on low sodium diet (like for heart problems)
should not be drinking water coming through this kind of water softener.
Con's: This water softener does not remove the salts (minerals),
it just makes them all sodium type of salts.
Con's: It does not remove most organic type of pollutants unless
they are positively charged.



As you can see there are some opposite views as to the validity of magnet therapy. But those who believe in magnet therapy will probably buy into the idea of magnetic water softening.
Here's a quote describing the book, Magnet Therapy:
"Magnet Therapy presents the history and science of this fascinating
subject, explaining in clear language why magnets increase oxygenation
in the blood, reduce cholesterol levels and blood pressure, reduce pain,
enhance cellular regeneration, and may even inhibit tumor growth."
Here's a quote describing the other book, Bad Medicine:
"The idea of bloodletting or shock therapy as cures for what ails
you seems antiquated and foolhardy. However, Wanjek believes that many
people believe in and practice equally bogus medical treatments today.
He debunks many common medical myths and misconceptions, including touch
therapy, magnet therapy, and the use of shark cartilage to ward off cancer."


Here's a quote from the site who sells these products. "Magnetically treated water has been used in Europe and Asia for many years. In the past 10 years this “new” idea has become widespread in the United States. It requires no pipe cutting, electricity or maintenance and lasts virtually forever. Just place the units over the pipe and attach with the included plastic ties.When water is magnetically charged, it electrically takes on a greater ionic charge than the minerals, which creates a natural magnetic attraction between the two. The magnetization then attracts and locks the dissolved minerals into the water creating healthy and cost-free de-scaling. Softening and better tasting water are the outcome of an actual reduction in the size of the water molecules. The small, magnetized water molecules have a greater solvency and a magnetic attraction that results in cleaner bathing and washing, and dramatically reduces the gases and foul taste of sulfur, chlorine, fluoride, etc."
A common approach of all these pseudoscience products
is the use of technobabble (technical descriptions that are meaningless
and have no basis in real science) and wonderful claims (Note words
like: wide-spread use, easy, lasts forever, no maintenance, healthy, cost-free,
dramatic.) They often succeed in selling these products for these reasons:
1) Most people are ignorant of physics and chemistry.
2) People love things that are cheaper and easier.
3) People want to believe what others are saying. Especially when they
are saying such wonderful things.
4) The saying, "A fool and his money are easily parted."
is sadly true.
5) People are generally too lazy to check things out for themselves
to see if they are valid.
6) When something works, people often jump to the conclusion of why it
worked. The credit is often given to something that had nothing to do
it.
Before we move on let's analyze some of the technobabble used to promote the product.
"When water is magnetically charged, it electrically takes on a greater ionic charge than the minerals, which creates a natural magnetic attraction between the two."
They are confused about magnetism and electrical charge. "Magnetically charged" makes no sense because charge comes from only electrons or protons, not magnetism. Have you ever heard of someone using a magnet for a battery? Again, by saying "a greater ionic charge...creates a natural magnetic attraction..." they are still confused about these two entirely different forces. Plus the only way water can have "a greater ionic charge" is for it to absorb an acid (a positive hydrogen ion, which doesn't come from magnets, only acids). But even then it would repel the positive metal ions (because like charges repel) and not "create a natural magnetic attraction."
Just reading this technobabble is annoying and painful to us who understand chemistry and physics.
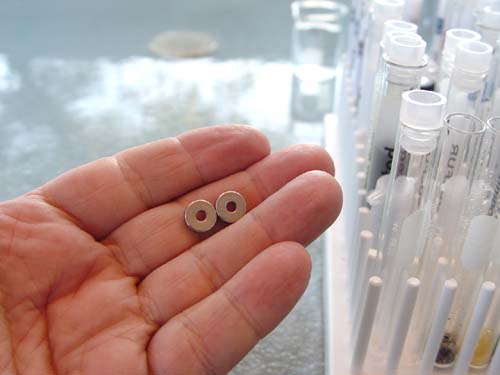

#1, 2, &3: You will be using both Ion Exchange Resins (Sodium type and Deionizing type). You will also use the activated charcoal.


#5. You will be using the small 50 mL beaker for holding the tap water.


#8: You will be using the Color Chart..

Fill the small 50mL beaker with tap water.



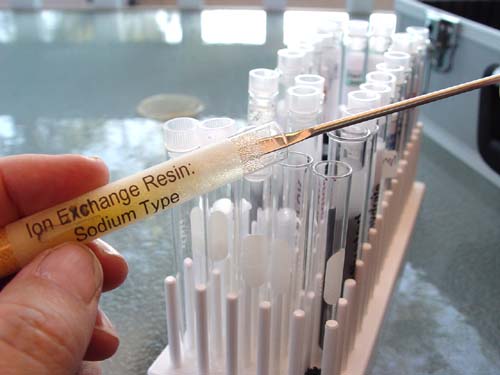

The Ion Exchange Resin Deionizing type will also require a microspatula to get it out. Place it in the middle test tube. Again, the caps to the other test tubes should be on so some of this doesn't fall into the wrong tube. That was the mistake I made, and it took a while to fish out the stray particles.

Drop the two magnets into the second test tube from the LEFT.
The far left test tube is left empty.
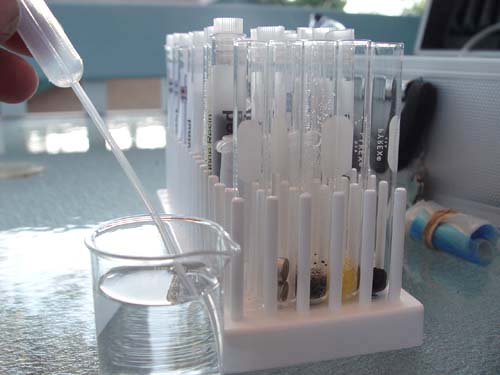

After the tap water has been added, you should have this line up. From Left to Right. Tap water only, magnets, ion exchange resin (deionizing type), ion exchange resin (sodium type), and activated charcoal.




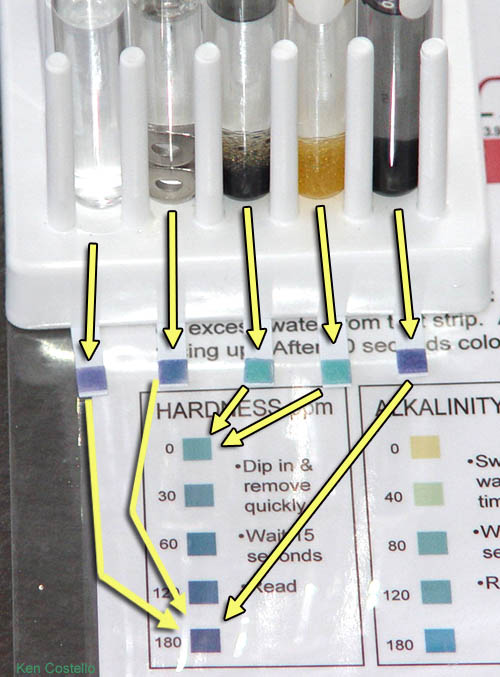
HARDNESS Results : Here I have the results from all 5 test tubes (remember you probably only have enough to do the right four.)
The results show that the two ion exchange resins brought the hardness to zero. The water in the other test tubes all read the same as the original tap water.
Remember hardness is measuring the amount of calcium and magnesium ions in the water. These are the ions that bind to soap and keeps it from making suds and available for cleaning. Calcium bound to soap comes out of solution and forms soap scum.
 (Take
a picture of your test tubes at this point in the lab.) Also report your
values to me when you send the picture.
(Take
a picture of your test tubes at this point in the lab.) Also report your
values to me when you send the picture.

The activated charcoal didn't help in reducing the metal ions calcium and magnesium. Of course, it was not expected to help. These ions are too small for the crevasses in activated charcoal to hold. But large compounds can be captured with activated charcoal.
Food coloring contains dyes from plants or animals. These are usually large organic compounds. You can use the food coloring that came with your kit to test activated charcoals ability to absorb it. Fill your small beaker with distilled water and then place two or three drops of the dye in the water. Notice I placed a saucer under the beaker to protect the counter top.
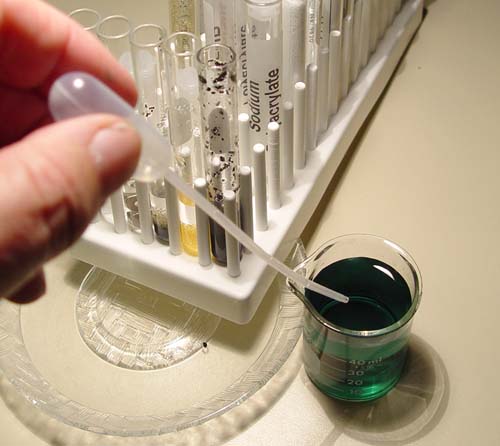
Now add about 3 or 4 drops of the colored water to each of the test tubes. Remember, you may have a different color than shown here.
Trivia: There are only 9 color additives certified to be used in food for humans in the United States.




Using a plastic pipette I transfered some of the liquid from the activated charcoal test tube to the glass pipette sitting in another test tube.



 (Take
a picture of your test tubes)
(Take
a picture of your test tubes) Here is the final line up. The test tube with only tap water is still greenish as expected. The water with the two magnets still has some green tint to it, so magnets apparently don't absorb organic molecules. The middle test tube with the dual ion exchange resin cleared up the water nicely. The ion exchange resin (sodium type) did not absorb this organic compound. The activated charcoal did. So what does this tell us? Being absorbed by the activated charcoal means that it is a relatively large organic (carbon-based) compound. The behaviour with the two resins indicate that the green food coloring molecule is probably negatively charged. Remember, the sodium ion exchange resin absorbs positive metal ions like calcium+2. It would also absorb any positively charged organic compound. But since it didn't, we can assume the green dye is not positive. The dual resin, however, can absorb both negatively and positively charged compounds.
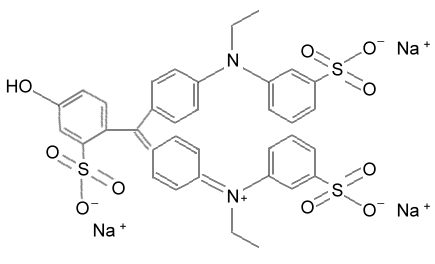
In water the three sodium ions (Na+) will get carried away by water molecules. The main structure has three SO3- groups (3 negative charges) and one positive charge on the lower nitrogen atom (N+). Overall the molecule would have a net charge of a negative 2 [(-3) + (+1)=(-2)]. That it why the sodium ion exchange resin did not trap it, but the dual resin did.
![]() (As
always, I need a picture of you doing some step in this lab. Perhaps
take a picture of you shaking a test tube or holding up a test tube
and looking at it.)
(As
always, I need a picture of you doing some step in this lab. Perhaps
take a picture of you shaking a test tube or holding up a test tube
and looking at it.)
Email the results you got to me along with
the three pictures from this lab.