Carbon Dioxide: Experiment #5: Ocean Acidity.
Demonstration of acidity caused by carbon dioxide

The bad news, good news and more bad news about elevated
CO2 levels in the atmosphere.
Bad news: CO2 contributes to global warming.
Good news: The ocean is absorbing a good deal of this CO2, therefore
slowing global warming.
Bad news: As the excess CO2 dissolves in the ocean, the ocean becomes
more acidic, therefore threatening marine life that uses calcium carbonate
to build its skeleton or shell. Think of coral, lobsters, star fish, and
mollusks.

When CO2 dissolves in water, we showed that it becomes carbonic acid.


There are many ways humans have damaged marine life; for example, pollution, over fishing, dredging, and blasting are the obvious ways. Adding carbon dioxide to the atmosphere is a more hidden way of harming marine life; a way that slowly dissolves their bodies.

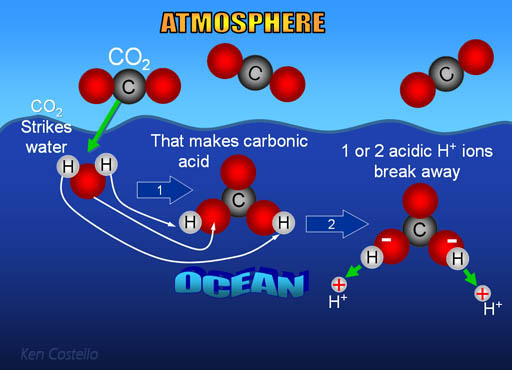
First carbon dioxide in the atmosphere combines with ocean
water to make an acid called carbonic acid (note: some CO2 dissolves in water without combining with water).
CO2 + H2O -> H2CO3
Like most acids, carbonic acid releases a hydrogen ion
(H+). It’s called an ion because it has a charge. Here it's a plus
one charge. The hydrogen ion (H+) is the
active ingredient of acids. H+
is a proton, so it's small and powerful.
H2CO3 -> HCO3- + H+
some HCO3--> CO32- + H+


(1) H+ ions (protons) released by carbonic acid hitch a ride on water molecules and then get attracted to the negative charges on the carbonate (CO32-). (2) the protons break away from the water and slide into the calcium carbonate getting in between the positive calcium and negative oxygen atoms. (3) The calcium ion (Ca2+) get replaced by the protons (H+ ions). (4) The displaced calcium ion gets surrounded by water. (5) The small protons continue to slip in and displace the calcium ions. (6) The carbonate with a proton attached (together they are called bicarbonate) is also surrounded by water. The outcome is calcium carbonate dissolves, and the marine animal looses its shell or exoskeleton and dies.
CaCO3(s) + H+(aq) --> Ca2+(aq) + HCO3-(aq)

To demonstrate that CO2 makes water acidic, we will bubble CO2 through water. Instead of the source of CO2 being your automobile, it will be yourself. Yes, you contribute to global warming and to the oceans acidity, too (but to a much lesser amount than your automobile).

We will detect the CO2 in two ways. Both ways show that the acidity of water increases when CO2 is added. One way of showing it is by directly measuring pH. The other way is the same as we did in the car exhaust lab, but this time, you will provide the CO2.


Air Stone:
We will begin with the measurement of pH after blowing CO2
through water. Locate the air stone in your kit. It is a porous stone
that allows air to flow through it while it breaks the air up into tiny
bubbles. This helps the gases in the breath dissolve more in the water. Note: Some air stones in the kits are black and spherical.

Attach Air Stone to Tubing:
Your kit will have a piece of blue tubing. Attach the air stone to the
blue tubing.
(There's also blue tubing on the portable air pump, but you should use the loose segment of tubing.)

Gases Dissolve better in Cold Water:
The amount of CO2 that dissolves in water depends on the temperature of
water. You know this from experience. If you open a soda can while warm,
it’s likely to spray all over as the CO2 hurriedly leaves the water
to become a gas. If cold, more CO2 stays dissolved in the water as either
CO2 or as H2CO3 (carbonic acid).

Place Ice and Water in Beaker:
So to get more of the carbon dioxide from our breath to
dissolve in the water, we are going to blow into a beaker of ice water
using some tubing attached to an air stone. The concentration of CO2
in air from our lungs is about 4% (4 CO2 molecules with the
remaining 96 being oxygen, water, and nitrogen). With car exhaust, CO2
concentration is about 12%.

Place Air Stone with Tubing into Beaker:
Place the air stone into the water and it should be submerged in the water.

Blow into tubing:
Blow into the tubing to get bubbles coming out of the air stone. It does take a bit of force to keep the air flowing. If you are asthmatic or have some lung problems, you might get someone else to do that for you.
Continue blowing for about 3 minutes. It's OK to take some short breaks during that time.

Use pH test strip:
You can use either pH test strip to see if the water has become acidic. The 0-14 pH range paper covers the whole range and the pH 4.0-7.0 test papers work because that's the range that a weak acid like carbonic acid could fall within.

Dip pH test strip into the Water:
Here I'm using the 0-14 pH range paper,
but you can use the 4.0-7.0 range. When you dip the water in, you need
to check it against the color chart quickly because carbonic acid will
decompose back to carbon dioxide and water pretty quickly. That means
the water on the test strip may be acidic (about a pH of 5.0) and in about
10 seconds it can be neutral as the carbonic acid decomposes into water
and carbon dioxide.

Check pH paper with Color Chart:
Here I found out the water had a pH between 5 and 6.
That means there's about 10 (pH6) to 100 (pH5) times more H+ ions (acid) in the water
after I blew carbon dioxide into it than when it started as neutral tap
water (which would have been pH 7).
Record and report what you get here.

Indirect detection of acid:
The above procedure was to create carbonic acid using our breath and to
measure the level of acid produced. Carbonic acid is a weak acid and our
breath is only 4% carbon dioxide, so our results there are not always
convincing. Another method is to use H+ ions in the carbonic
acid to neutralize OH- ions that are in alkaline solutions.
If we use an indicator that changes color with a different pH, our results
can be more convincing.
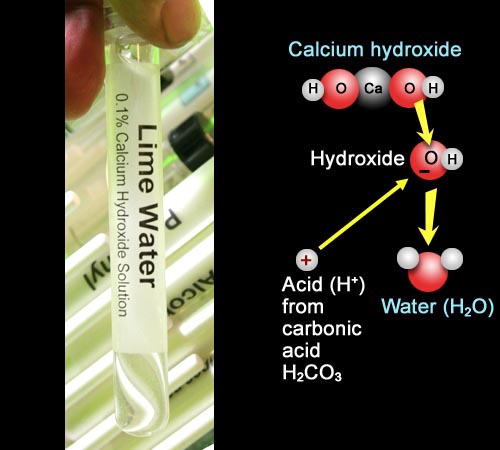
Just like we did in the Car Exhaust experiment, we will use some lime water (saturated calcium hydroxide) as our alkaline solution. The reaction shows that the acid from the carbonic acid will combine with the hydroxide ions (OH-) to form water.

Measure 3mL of water:
Use your Purified Water bottle and the small graduated cylinder to measure out 3 milliliters of water. This is the same thing you did with car exhaust lab.

Add 1 mL of lime water:
Use a clean plastic pipette to transfer about 1 milliliter of lime water (calcium hydroxide solution) to the graduated cylinder bringing the level up to the 4mL mark.

Pour diluted lime water into beaker:
Pour the four milliliters of the diluted lime water into the small 50 mL beaker.

Add Phenolphthalein:
Add about 2 to 4 drops of your phenolphthalein solution (made in Exp 2) to the diluted lime water. The solution should turn a dark pink. If it doesn't then the lime water may have gotten neutralized by CO2 in the air. If the cap to the test tube with the lime water isn't air tight, air can get in. The CO2 in the air will neutralize the lime water's OH- ions. In that case, find the test tube with sodium bicarbonate, and add a few grains of the powder to this solution. Stir it and see if it turns pink. If not, add a few more grains of sodium bicarbonate. Sodium bicarbonate produces some OH- ions in the water.
Phenolphthalein is pronounced feenol-thay-leen.

The phenolphthalein we added will have two structures.
When clear it is the left structure. It becomes the right structure as
it turns pink in the lime water (Ca2+ and OH-).
What happens is these negative hydroxide (OH-)
ions pull off the hydrogen atoms to form water (H2O) See red
arrows. When the hydrogen atoms are pulled off, they leave their electrons
behind becoming H+ ions. The extra electrons cause a shifting
of electrons that generates the right-side structure. One outcome is the
central carbon (blue arrow) gets connected
to the upper left ring by sharing two electrons (called a double bond
and indicated with a double line). In the right structure notice the double
bonds (double lines) alternate with single bonds (single lines). This
allows electrons to jump around the whole structure. This free movement
of electrons allows them to absorb various frequencies of light. The solution
is pink because it is absorbing most colors but lets pink color pass through.
 Take
a picture of your solution at this point.
Take
a picture of your solution at this point.

Blow into Beaker:
As you blow into the beaker you will be introducing carbon dioxide
that will form carbonic acid which breaks up into H+ ions and
carbonate ions CO32-. The H+ ions are
going to neutralize the OH- hydroxide ions of the lime water.
After that, the H+ ions are going to attach to the phenolphthalein
turning it back to its clear form. It might take 5 minutes of blowing. So you might want to trade off with someone. If you start to get dizzy realize that's because you are losing too much CO2 from your blood stream, which is removing H+ ions from your blood. So as the pink solution is becoming more acidic, your blood is becoming less acidic. That creates the condition called respiratory alkalosis. So take some breaks if you feel dizzy. If it doesn't change after 5 minutes, there may have been too much sodium bicarbonate added. If so, just report that it never changed. No need to get overly dizzy.
 Take a picture of yourself holding the beaker either before OR after it turns from pink to clear.
Take a picture of yourself holding the beaker either before OR after it turns from pink to clear.
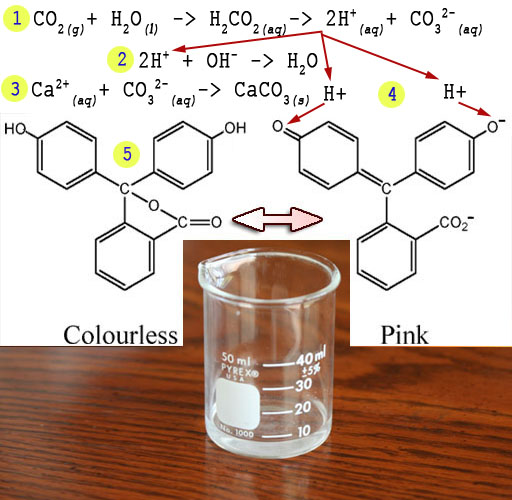
Here are
the steps.
1)
CO2 combines with water to make acid (H+) and carbonate
(CO32-).
CO2(g)
+ H2O(l) > H2CO3 >
CO32-(aq) +2H+(aq)
2) The acid neutralizes the OH- from the lime water.
2H+(aq)
+ OH-(aq) > H2O(l)
3) The carbonate and calcium combine to make chalk
CO32-(aq)
+ Ca2+(aq) > CaCO3(s)
4) Additional H+ created attaches to the phenolphthalein returning it to its clear form (5).
Note: (g)=gas, (l)=liquid, (aq)=aqueous, i.e., dissolved in water, (s)=solid.

The changing of dark pink to clear is an indicator that
carbon dioxide from your breath entered the water, turned to acid, neutralized
the alkaline solution, and returned the phenolphthalein to its clear form.